• For a reaction to take place;
– the reacting molecules should collide.
– the colliding molecules should have a proper orientation.
– the reacting molecules should have kinetic energy greater than or equal to certain minimum value. This minimum value of energy is called activation energy.
Consider a simple reaction involving a collision between two molecules – ethene, CH2=CH2, and hydrogen chloride, HCl, for example. These react to give chloroethane.
![]()
![]()
As a result of the collision between the two molecules, the double bond between the two carbons is converted into a single bond. A hydrogen atom gets attached to one of the carbons and a chlorine atom to the other.
The reaction can only happen if the hydrogen end of the H-Cl bond approaches the carbon-carbon double bond. Any other collision between the two molecules doesn’t work. The two simply bounce off each other.
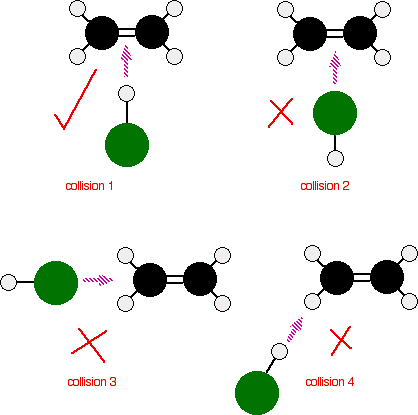
Of the collisions shown in the diagram, only collision 1 may possibly lead on to a reaction.
collision 2 won’t work as well. The double bond has a high concentration of negative charge around it due to the electrons in the bonds. The approaching chlorine atom is also slightly negative because it is more electronegative than hydrogen. The repulsion simply causes the molecules to bounce off each other.
Even if the species are orientated properly, you still won’t get a reaction unless the particles collide with a certain minimum energy called the activation energy of the reaction.
Activation energy is the minimum energy required before a reaction can occur. You can show this on an energy profile for the reaction. For a simple over-all exothermic reaction, the energy profile looks like this:
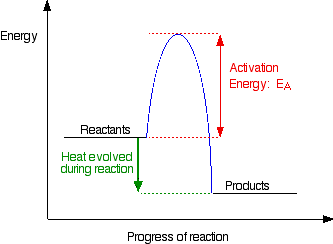
If the particles collide with less energy than the activation energy, nothing important happens. They bounce apart. You can think of the activation energy as a barrier to the reaction. Only those collisions which have energies equal to or greater than the activation energy result in a reaction.
Any chemical reaction results in the breaking of some bonds (needing energy) and the making of new ones (releasing energy). Obviously some bonds have to be broken before new ones can be made. Activation energy is involved in breaking some of the original bonds.
Where collisions are relatively gentle, there isn’t enough energy available to start the bond-breaking process, and so the particles don’t react.
• The rate of reaction depends on the activation energy (Ea). When Ea decreases, the number of molecules which have higher energy than Ea, increases. So, the number of effective collisions increases and the rate is increased.
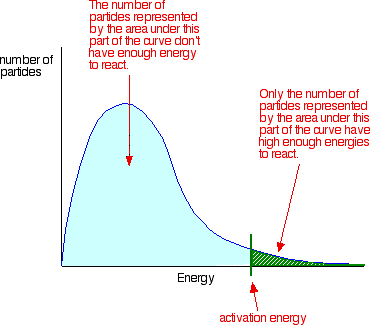
Notice that the large majority of the particles don’t have enough energy to react when they collide. To enable them to react we either have to change the shape of the curve, or move the activation energy further to the left.
When temperature increases, the kinetic energy and the number of collisions per unit time and per unit volume also increase. Hence, the number of effective collisions per unit time also increases. Thus the rate of reaction increases. The fraction of molecules having energy greater than the activation energy increases rapidly in most reactions even with a small increase in temperature. This can be explained by the Maxwell -Boltzmann energy distribution graphs.
the graph labelled T is at the original temperature. The graph labelled T+t is at a higher temperature.
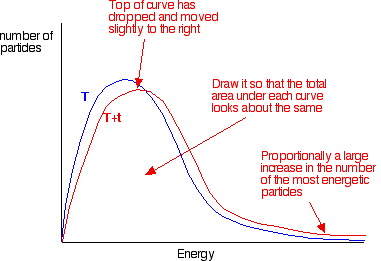
If you now mark the position of the activation energy, you can see that although the curve hasn’t moved very much overall, there has been such a large increase in the number of the very energetic particles that many more now collide with enough energy to react.
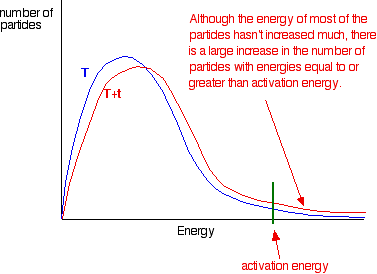
The area under a curve gives a count of the number of particles. On the last diagram, the area under the higher temperature curve to the right of the activation energy looks to have at least doubled – therefore at least doubling the rate of the reaction.
When the concentration of the reactants increases the number of molecules per unit volume increases. Hence, the number of effective collisions per unit volume and per unit time also increases. So, the rate of reaction increases.
Note-In gaseous reactions, when the pressure increases at a constant temperature, the volume decreases. So the concentration increases. In gaseous reactions, the rate of reaction increases with the increasing pressure.
To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy.
In other words, to move the activation energy on the graph like this:
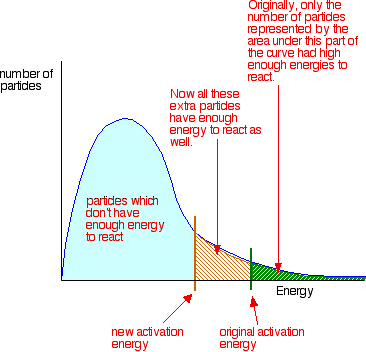
Adding a catalyst has exactly this effect on activation energy. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. Showing this on an energy profile:

The smaller the size of solid reactant particles, the greater the surface area with which the reacting molecules can collide. This increases the reaction rate.
e.g. Powdered CaCO3 reacts faster than lumps of CaCO3 with a solution of HCl.
