• Polymers are large molecules formed by the union of a large number of repeating units.
eg. n(C2H4) Polymer
Polymers can be classified as natural and synthetic.
• Natural polymers are naturally synthesised in living/bio systems.
eg. Natural rubber, proteins enzymes.
• Synthetic polymers are synthesised by man (man made polymers).
eg. Polythene, polyvinyl chloride, polyesters, Teflon, Bakelite, nylon,urea formaldehyde.
Polymers can be classified in accordance with the method of synthesis.
• Addition polymers
• Condensation polymers
Polymers can be classified based on their thermal properties.
• Thermoplastic polymers :
Can be softened by heating, allow to cool and harden and then re-softened many times. The forces of attraction between chains are weak.
eg. Polythene, PVC, Polystyrene
• Thermosetting polymers:
During the first stage of manufacture, once moulded they set and then cannot be re-softened by heating. Polymer chains are cross linked to form a three dimentional structure.
eg. Bakelite, Urea-formaldehyde
Unsaturated monomers undergo additional polymerization to form addition polymers.

Polythene is a tasteless, odourless, light, non-poisonous, relatively cheap, thermoplastic polymer. It is used to produce packing materials, trash bags, seat covers, bottles,various containers, toys etc.
• Although PVC is a thermoplastic, due to the presence of chlorine, PVC does not catch fire easily. Its attractive forces among the chains are stronger than those in polyethylene and therefore is more harder. A special feature of PVC is that it can be processed with stablisers and fillers making it versatile. PVC is used to make water pipes, gutters, wire insulations, films, floor covers, seat covers, raicoat covers, umbrella materials, etc.
• The monomer is styrene.
• Polystyrene is a transparent substance and a thermoplastic. Made into foam and solidified, it can be used as a insulation and packing material popularly known as ‘Rigifoam’.
• Tetrafluoroethene is the monomer.
• Although it is a thermoplastic due to the presence of halogen it can withstand high temperatures. Therefore, it is used in making fire proof clothing. In addition Teflon is not wettable and therefore, it is used as coatings in non-stick cooking vessels. Being chemically inert, teflon can resist almost all corrosive chemicals, so it is used in valves, seals, gaskets etc. in plants used in chemical industry.
• Rubber is used to make tyres, tubes, mattresses and gloves used in the medical field.
Natural rubber vulcanization
• Presence of cis-polyisoprene chains is the reason for elasticity of rubber. In order to change the elastic nature of rubber as required and to make it stronger, it is heated with 1% – 3% sulphur by weight. This is called vulcanisation. Vulcanisation makes cross links among polyisoprene chains through sulphur bridges and more resilient and tougher.By heating with 25% – 35% sulphur, ebonite is obtained. The vulcanized rubber is not sticky and has superior mechanical properties such as higher elesticity.
Rubber compounding
Natural rubber or other rubber itself may not be a useful material for a particular purpose.But, based on the material mixed, rubber compounding makes it a useful material with desired properties. Material used in rubber compounding can be classified by the function they serve. Main functional cases are given below.
• Elastomers
• Vulcanizing or cross linking agents
• Accelerators
• Activators / retarders
• Process aids
• Softeners and plasticizers
• Reinforces / Fillers
• Age resistors
For example, cover of a conveyor belt is made of the following compounds.
Natural rubber (Elastomer)
Carbon black (Filler)
ZnO (Accelerator)
Stearic acid
Rubber process oil
Resin
N-Cyclohexylbenzothiozole-2-sulfonamide (CBS)
Sulphur (Vulcanizing agent)
The polymers formed by the joining of monomers with elimination of small molecules such as H2O, NH3 or HCl are called condensation polymers.
• Polymers formed by joining of monomers by – CONH – group are known as polyamides.
Nylons are polyamides. One of the commonest is nylon 6,6 which is made by condensation polymerisation between 1, 6 – diaminohexane and hexanedioic acid. The reaction is more effective when acid chloride is used instead of the dicarboxylic acid.

• Nylon is a thermoplastic polymer and it is used to produce fibres which have structural similarities to silk and wool. Despite its structural similarity to wool, nylon lacks the softness and the moisture – absorbing properties of the natural fibre. It is, however, harder wearing and has good ‘wash and wear’ characteristics. Nylon is mainly used to make soft and light clothing. Other uses include tufted carpets, tyre cords, machine gear wheels and bearings, fishing nets and non wetable tent covers. The elasticity and strength of nylon make it ideal for making stockings and tights.
• Polymers with monomers linked by the -COO- (ester) groups are commonly called
polyesters.
Terelene formed by the condensation polymerisation between ethane -1,2-diol(ethylene glycol) and benzene – 1,4 – dicarboxylic acid (Terephthalic acid) is an example for a polyester.

• Terelene is a thermoplastic. It is used as a substitute for natural fibres such as cotton and wool. Being strong, the fibres are used in the manufacture of fibre glass. It is also used in the production of textiles, photographic films and audio tapes.
In the presence conc. H2SO4 phenol and formaldehyde (methanal) react to form a thermosetting polymer know as Bakelite.
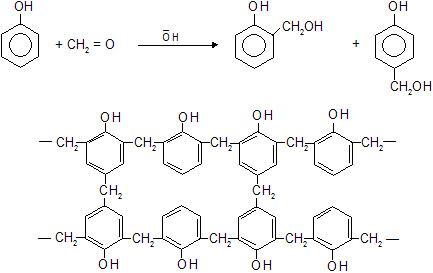
• Bakelite is a cross linked three dimensional polymer. These cross links lead to a very rigid structure because the various groups are not free to twist round and move their positions. Bakelite is used to make heat resistant parts of electric utensils.
In the presence conc. H2SO4 urea and formaldehyde react to form a thermosetting polymer know as urea-formaldehyde.
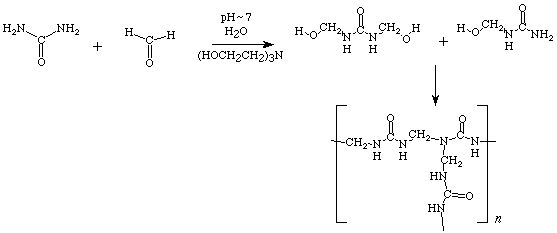
• Urea-formaldehyde is a cross linked three dimensional polymer. Ther are used as thermosetting plastic or adhesives.
