• The International Union of Pure and Applied Chemistry -IUPAC has developed a method for systematically naming organic compounds.
• The name given to a compound according to the IUPAC nomenclature consists of several parts.
1. The suffix that is used to indicate the main functional group of the structure
2. Name of the chain that is used to identify the main carbon chain of the compound
3. The prefixes that are used to indicate the substituent groups
4. The numbers that are used to indicate the places at which the substituent groups, additional groups and the main functional groups are attached to the chain
• The IUPAC name of an aliphatic compound can easily be developed by following the steps stated below in the given order.
1. Identifying the principal functional group
2. Selecting the main chain
3. Selecting the root name for the principal chain
4. Addition of the suffix for the double/triple bond in the main carbon chain to the name of the chain
5. Addition of the suffix used to indicate the principal functional group to the name of the chain
6. Naming the substituent groups
7. Adding the names of the substituent groups to the name of the chain
8. Numbering the carbon chain
9. Writing the numbers that are used to indicate the positions of the main functional group and the substituent groups in front of these groups.
The series of functional groups arranged in the decreasing order of their priority

The root names used for the compounds according to the number of carbon atoms and the name of the corresponding hydrocarbon
| No. of Carbons |
CnH2n+2 |
||
| CH4 | CH4 | ||
| C2H6 | CH3CH3 | ||
| C3H8 | CH3CH2CH3 | ||
| C4H10 | CH3CH2CH2CH3 | ||
| C5H12 | CH3CH2CH2CH2CH3 | ||
| C6H14 | CH3CH2CH2CH2CH2CH3 | ||
| C7H16 | CH3CH2CH2CH2CH2CH2CH3 | ||
| C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 | ||
| C9H20 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | ||
| C10H22 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 |
Drawing the structural formula of a compound according to the IUPAC nomenclature
• Drawing the structural formula of a compound according to the IUPAC nomenclature can be done by following the steps given below.
1. Identifying the chain and drawing the chain according to that name
2. Numbering the chain
3. Identifying the principal functional group and the remaining groups according to the IUPAC name given and joining these groups to the correct places of the chain according to the number in front of these groups
4. Placing hydrogen atoms in the chain structure so that each carbon atom has a valency of four
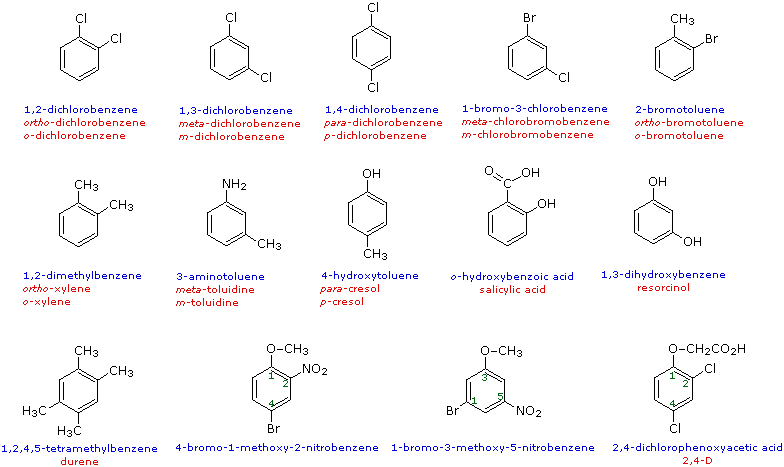
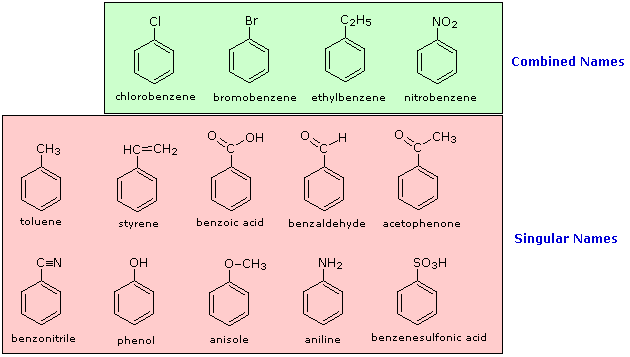
The nomenclature of substituted benzene ring compounds is less systematic than that of the alkanes, alkenes and alkynes. A few mono-substituted compounds are named by using a group name as a prefix to “benzene”, as shown by the combined names listed below. A majority of these compounds, however, are referred to by singular names that are unique. There is no simple alternative to memorization in mastering these names.
When more than one substituent is present on a benzene ring, the relative locations of the substituents must be designated by numbering the ring carbons or by some other notation. In the case of disubstituted benzenes, the prefixes ortho, meta & para are commonly used to indicate a 1,2- or 1,3- or 1,4- relationship respectively. In the following examples, the first row of compounds show this usage in red. Some disubstituted toluenes have singular names and their isomers are normally designated by the ortho, meta or para prefix. Finally, if there are three or more substituent groups, the ring is numbered in such a way as to assign the substituents the lowest possible numbers, as illustrated by the last row of examples. The substituents are listed alphabetically in the final name. If the substitution is symmetrical (third example from the left) the numbering corresponds to the alphabetical order.