There is a loosely bound cloud of electrons on both faces of the planar benzene molecule. As with alkenes, this makes benzene reactive towards electrophiles. The first step in this reaction is for the electrophile (E+) to form a bond with a carbon atom in the benzene ring.
The intermediate carbocation so formed is stabilized by the delocalization of the positive charge by conjugation with the two π bonds. This can be shown by resonance.
However, in going from benzene to the carbocation the cyclic delocalization of π electrons is broken, and the aromatic stabilization energy is lost. Therefore, the intermediate carbocation prefers to lose a proton and re-established the cyclically delocalized electron cloud, rather than reacts with a nucleophile and give an addition product as in the case of alkenes.
The proton is usually taken up by one of the bases (B:–) present in the reaction mixture. Thus, the result is the substitution of a H atom on the benzene ring with E.
In the presence of the nitration mixture composed of conc. HNO3 and conc. H2SO4, nitrobenzene is formed by the substitution of H by a nitro group.
The electrophile here is +NO2 generated in the medium by the dehydration of nitric acid by sulphuric acid.
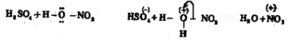 The base which takes up the proton in the final step is HSO4–.
The base which takes up the proton in the final step is HSO4–.
During the reaction of benzene with alkyl halides in the presence of a Lewis acid like anhydrous AlCl3, substitution by an alkyl group takes place.
The electrophile here is R+.
R-Cl + AlCl3 → R+AlCl4–
In cases where R+ is not very stable (eg. +CH3) the species actually reacting with the benzene molecule may be a R-Cl molecule polarized by coordination to AlCl3, which will transfer R+ to the benzene molecule during the reaction by cleavage of the R-Cl bond.
The base which takes up the proton in the final step is AlCl4–
AlCl4– + H+ → AlCl3 + HCl
During the reaction of benzene with acid chlorides in the presence of a Lewis acid like anhydrous AlCl3, substitution by an acyl group takes place
The electrophile here is R-C+=O.
The base which takes up the proton in the final step is
AlCl4– + H+ → AlCl3 + HCl
When benzene reacts with halogens (Cl2 or Br2) in the presence of a Lewis acids (such
as FeCl3, AlCl3) substitution by a halogen group takes place in the benzene ring.
The efective electrophile here is Cl+. It is transferred to the benzene ring from the complex AlCl4– during the reaction.
AlCl3 + Cl2 → Cl3Al––+Cl-Cl
The base which takes up the proton in the final step is AlCl4– .
AlCl4– + H+ → AlCl3 + HCl
Benzene does not get oxidized by normal oxidizing agents like H+/KMnO4. However, the alkyl group in alkyl substituted benzene can be oxidized by H+/KMnO4 to a carboxylic acid group. The benzene ring does not oxidize easily due to its stability. H+/K2Cr2O7 can also be used for this reaction.
Tertiary alkyl groups do not get oxidized under the conditions in which primary and secondary alkyl groups get oxidized. More vigorous conditions under which tertiary alkyl groups can be oxidized also result in cleavage of the benzene ring.
Although benzene does not undergo electrophilic addition reactions, like alkenes, they can add hydrogen in the presence of suitable catalysts. The temperatures used are higher than for alkenes.
