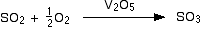• Rate of a reaction can be defined as the change of concentration (either decrease of
concentration of reactants or increase of concentration in products) within a unit time.
• Factors affecting rate of reactions
– Temperature
– Concentration (Pressure)
– Physical nature (Surface area of reactants)
– Catalysts (Heterogeneous/Homogeneous)
• Equal volumes of water (10.0 cm3) are added to test tubes along with two pieces of magnesium ribbon of 2 cm long. Apparatus are arranged as in the diagram keeping all the factors identical except the concentration of an acid by adding 2 drops of a hydrochloric acid solution to one test tube and 4 drops of the same solution to the other.
• Gas bubbles evolve faster in the test tube containing acid in higher concentration.
• It can be concluded that concentration affects the rate of a reaction.
• Apparatus are set as shown in the diagram keeping all the factors identical except
temperature.
• It can be seen that the solution kept at higher temperature decolourizes faster.
• Therefore, temperature affects the rate of a reaction.
• To two test tubes separately add a lump of CaCO3 and CaCO3 powder of equal mass.
• To each test tube, add equal volumes of a HCl solution of the same concentration (e.g.0.01 mol dm-3)
• Keep both tubes in a water bath.
• Observe the difference in the rates of evolution of gas.
• Emphasize the need to immerse in a water bath.
• It can be observed that the tube containing CaCO3 powder effervesces faster.
• Thus, it can be concluded that the physical nature of reactants affects the rate of a reaction.
• Add 10.0 cm3 samples of ’20 volume’ H2O2 to two boiling tubes. (’20 volume’ means that unit volume of H2O2 solution gives twenty volumes of O2 at STP)
• Add 5.0 cm-3 of water to one tube and 5.0 cm3 of 0.1 mol dm-3 NaOH to the other.
• It is seen that gas bubbles evolve faster in the tube with NaOH.
• This leads to the conclusion that NaOH increases the rate of decomposition of H2O2.
(a) Homogeneous catalysts : If the catalyst and the reactants are in the same phase, they are called homogeneous catalysts.

(b) Heterogeneous catalysts : If the catalyst and the reactants are in different phases, they are heterogeneous catalysts.