Elementary reactions are classified according to their molecularity. The number of reacting species, which are involved in simultaneous collision to bring about a chemical reaction is called the molecularity of the reaction. In a unimolecular reaction, a single molecule shakes itself apart or its atoms into a new arrangement.
Example
Decomposition of NH4NO2 to N2 and 2 H2O

Bimolecular reactions involve the collision of pair of molecules. Bimolecular reactions are common.
Example :
Oxidation of nitric oxide by ozone

Intermolecular reactions, three molecules collide simultaneously. Such reactions are rare.

Molecularity is defined for elementary reactions where as order corresponds to the overall reaction. The overall reaction might involve one elementary reaction or it might involve a sequence of elementary reactions (a sequence of elementary reactions denotes a complex reaction).
The rate determining step
The overall rate of a reaction (the one which you would measure if you did some experiments) is controlled by the rate of the slowest step. In the example above, the hydroxide ion can’t combine with the positive ion until that positive ion has been formed. The second step is in a sense waiting around for the first slow step to happen.
The slow step of a reaction is known as the rate determining step.
As long as there is a lot of difference between the rates of the various steps, when you measure the rate of a reaction, you are actually measuring the rate of the rate determining step.
Example
By doing rate of reaction experiments, you find this rate equation:
![]()
![]()
The reaction is first order with respect to the organic compound, and zero order with respect to the hydroxide ions. The concentration of the hydroxide ions isn’t affecting the overall rate of the reaction.
If the hydroxide ions were taking part in the slow step of the reaction, increasing their concentration would speed the reaction up. Since their concentration doesn’t seem to matter, they must be taking part in a later fast step.
Increasing the concentration of the hydroxide ions will speed up the fast step, but that won’t have a noticeable effect on the overall rate of the reaction. That is governed by the speed of the slow step.
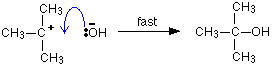
This is much easier to talk about with a real example. The equation below shows an organic chemistry reaction in which a bromine atom is being replaced by an OH group in an organic compound. The starting compound is bromoethane, and the organic product is ethanol.
![]()
![]()
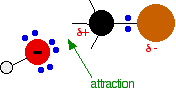
During the reaction one of the lone pairs of electrons on the negatively charged oxygen in the -OH group is attracted to the carbon atom with the bromine attached.
That’s because the bromine is more electronegative than carbon, and so the electron pair in the C-Br bond is slightly closer to the bromine. The carbon atom becomes slightly positively charged and the bromine slightly negative.
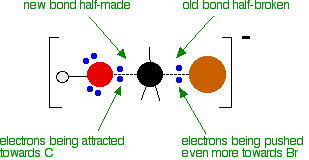
As the hydroxide ion approaches the slightly positive carbon, a new bond starts to be set up between the oxygen and the carbon. At the same time, the bond between the carbon and bromine starts to break as the electrons in the bond are repelled towards the bromine.
At some point, the process is exactly half complete. The carbon atom now has the oxygen half-attached, the bromine half-attached, and the three other groups still there, of course.
And then the process completes:
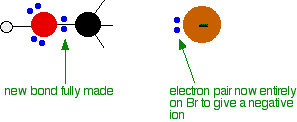
The second diagram where the bonds are half-made and half-broken is called the transition state, and it is at this point that the energy of the system is at its maximum. This is what is at the top of the activation energy barrier.
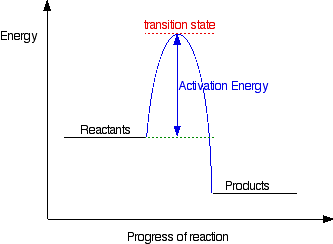
But the transition state is entirely unstable. Any tiny change in either direction will send it either forward to make the products or back to the reactants again. Neither is there anything special about a transition state except that it has this maximum energy. You can’t isolate it, even for a very short time.
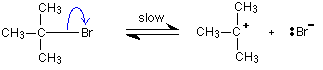
For reasons which you may well meet in the organic chemistry part of your course, a different organic bromine-containing compound reacts with hydroxide ions in an entirely different way.
In this case, the organic compound ionises slightly in a slow reaction to produce an intermediate positive organic ion. This then goes on to react very rapidly with hydroxide ions.


The big difference in this case is that the positively charged organic ion can actually be detected in the mixture. It is very unstable, and soon reacts with a hydroxide ion (or picks up its bromide ion again). But, for however short a time, it does have a real presence in the system. That shows itself in the energy profile.
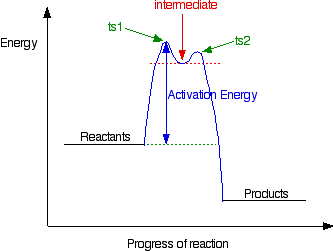
The stability (however temporary and slight) of the intermediate is shown by the fact that there are small activation barriers to its conversion either into the products or back into the reactants again.
Notice that the barrier on the product side of the intermediate is lower than that on the reactant side. That means that there is a greater chance of it finding the extra bit of energy to convert into products. It would need a greater amount of energy to convert back to the reactants again.
These peaks are labelled “ts1” and “ts2” – they both represent transition states between the intermediate and either the reactants or the products. During either conversion, there will be some arrangement of the atoms which causes an energy maximum .
