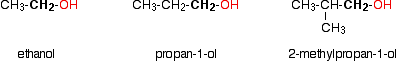
Monohydric alcohols can be classified into three types as primary, secondary and tertiary.
Primary alcohols
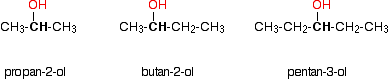
Secondary alcohols
Tertiary alcohols
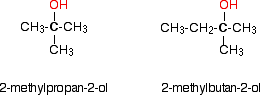
In alcohols the –OH bond is polarized as – R – O δ- -H δ+ . Hence, due to the inter molecular hydrogen bonds formed between alcohol molecules, their boiling points have higher values compared to the alkanes and ether with comparable relative molecular masses. The boiling point increases in going down the series of alcohols.
The above diagram shows how the inter- molecular hydrogen bonds exist in ethanol. Alcohols which have low relative molecular mass are soluble in water. The solubility of alcohols in water is due to the – OH group which can forms H – bonds with water molecules. The non polar alkyl group in the alcohol molecule is a hindrance to the solubility in water. In going down the homologous series of alcohols the size of the non- polar alkyl group gradually increases relative to the –OH group.
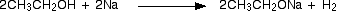
Alcohols behave as acids and react with sodium liberating hydrogen and forming sodium alkoxides. The alkoxide ion is a strong nucleophile and also a strong base.
Alcohols react with carboxylic acids to form esters. For this esterification reaction, concentrated H2SO4 acid acts as a catalyst.
The esterification reaction is both slow and reversible. The equation for the reaction between an acid RCOOH and an alcohol R’OH (where R and R’ can be the same or different) is:
![]()
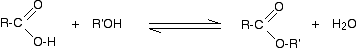
So, for example, if you were making ethyl ethanoate from ethanoic acid and ethanol, the equation would be:
![]()
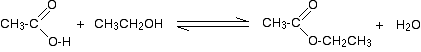
Alcohols react with PCl3 or PCl5 to give alkyl chlorides.
![]()
![]()
Alcohols under go nucleophilic substitution reaction with HBr to give the corresponding alkyl bromides. Protonation of the O atom converts the -OH group into a better leaving group.
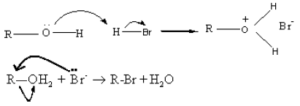 In this reaction Br- ion acts as a nucleophile and the leaving group is H2O.
In this reaction Br- ion acts as a nucleophile and the leaving group is H2O.
In this reaction, R-OH is converted to R-Cl. ZnCl2 is used as a catalyst. Because alkyl halides are insoluble in water, as the raction proceeds the reaction mixture becomes cloudy and turbid. The time taken for the turbidity to appear, after the mixing of reagents, can be used to distinguish between primary, secondary and tertiary alcohols.
Under the provided reaction conditions the above nucleophilic substitution reaction takes place in two steps. Tertiary alcohols form stable intermediate tertiary carbocations and therefore, tertiary alcohols in the presence of the Lucas reagent forms a turbidity in a very short time. Secondary alcohols take longer time and primary alcohols react very slowly.
Alcohols undergo an elimination reaction when treated with conc. H2SO4 or when heated with alumina to a higher temperature. The reaction in which a molecule of water is eliminated from an alcohol is the dehydration of alcohols. Here, an alkene is formed as the product of the reaction.
The product of oxidation depends on primary, secondary or tertiary nature of the alcohol. Oxidation of alcohols can be carried out with H+/KMnO4 or H+/K2Cr2O2 or H+/CrO3.
(i) Primary alcohols

In the presence of the above mentioned oxidizing agents primary alcohols first give aldehydes. These are further oxidized to carboxylic acids. If pyridinium chlorochromate [C5H5NH+CrO3Cl] is used, the reaction can be stopped at the stage where aldehyde is formed.
(ii) Secondary alcohols are oxidized to give ketones.
(iii)Tertiary alcohols
Normally the tertiary alcohols do not undergo oxidation under conditions that primary and secondary alcohols are oxidized.
