• In alkanes all the bonds are either C-C or C-H bonds. The polaritiy of those C-C and C-H bonds are low. Therefore, they do not react with common polar reagents (eg. OH– , CN– , H+ ) under normal conditions.
Alkanes react with the reagents such as Cl2 and Br2 that undergo easy homolytic cleavage to generate free radicals.
The mechanism of the chlorination of methane is given below.
Initiation step
Chain propagation steps
The Cl. free radical generated in the initiation step abstracts a H atom from the methane molecule by homolysis of the C-H bond.
The .CH3 free radical then reacts with a Cl2 molecule forming CH3Cl and generate
another .Cl free radical which can continue the chain reaction.
Chain termination steps
Combination of free radicals in the reaction mixture to form stable molecules, results in the termination of chains.
• The loosely bound π electron cloud which lies above and below the plane of the
ethylene molecule is capable of attracting electrophilic reagents.
• A molecule or an ion that can accept a pair of electrons is referred to as an electrophile.
• Here, the hydrogen atom which is the electron deficient pole of HBr molecule acts as an electrophile and attacks the double bond. During these electrophilic addition reactions, intermediate carbocations are formed.
Stability of carbocations follows the following order.
• When alkyl groups are attached to the positively charged C atom of the carbocation, the stability of the cation increases. The reason for this is the release of electrons by the alkyl groups through C-C σ bonds towards the positively charged carbon atom to which they are attached. This results in spreading the positive charge thereby stabilizing the ion.
• In the electrophilic addition reactions of hydrogen halides to asymmetric alkenes, two different carbocations can be formed after the bonding of the electrophile. Out of these the more stable carbocation forms more easily.
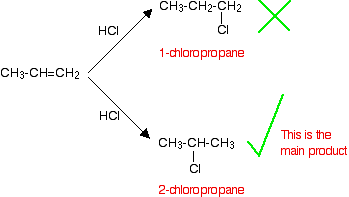
• The more stable carbocation is obtained when the electrophile gets attached to the carbon atom to which the highest number of hydrogen atoms are attached.
• After studying reactions of a large number of alkenes, this observation has been generalized as Markownikoff’s rule.
• Hydrogen bromide adds in the opposite way to this rule when there are peroxides in the reaction medium. The reason for this is that in the presence of peroxides the reaction of hydrogen bromide and the alkenes takes place via a free radical mechanism. It is not expected to describe this mechanism.
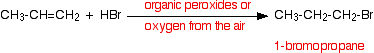
This change of the direction of addition (Anti-Markownikoff’s addition) is not exhibited by the other hydrogen halides.
Mechanism
• When a molecule of Br2 approaches the π electron cloud of an alkene molecule, it gets polarized . The positive end of the dipole then reacts with the alkene, transferring a Br+ ion to it during the reaction (by heterolytic cleavage of the Br-Br bond) forming a cyclic bromonium ion.
• In the second step of the reaction, a Br– ion acting as a nucleophile, forms a bond to one of the carbon atoms bonded to Br+ . The bond formed by that carbon atom to Br+ is broken during this step, giving an open chain structure again.
• Here, proton acts as an electrophile and HSO4– ion acts a nucleophile
![]()
![]()
eg-Ethene reacts with hydrogen in the presence of a finely divided nickel catalyst at a temperature of about 150°C. Ethane is produced.
![]()
![]()
• Alkynes have two π bonds which react independantly and undergo electrophilic addition reactions with reagents that add to alkenes.
• In the presence of Hg 2+ and dilute sulphuric acid, one molecule of water gets added on to alkynes.
• The rapid rearrangement of the enol to the aldehyde is due to the high stability of C = O.
• In the presence of catalysts such as finely powdered Pt, Pd or Ni alkynes react with hydrogen to give alkanes. The reaction can be stopped at the intermediate alkene stage by using a Pd/BaSO4 catalyst poisoned by quinoline.
• In the alkynes, H-C≡C-Hand R-C≡C-H the H attached to the C that forms the triple bond (terminal hydrogen) shows acidic properties. The acidic H in these alkynes can be displaced by metals.
