• Almost all cations form atoms in a flame test. The colour of the flame in the flame test is associated with elements possessing low energy gaps.
• The precipitates of the cations having d7, d8, d9 and d10 electronic configurations are soluble in excess ammonia and form the respective stable complex ions.
M2+(aq) + X2-(aq) → MX(s)
(d7) [Co(NH3)6]2+ – Yellow brown
(d8) [Ni(NH3)6]2+ – Deep blue
(d9) [Cu(NH3)4]2+ – deep blue
(d10) [Zn(NH3)4]2+ – colourless
(d10) [Ag(NH3)2]+ – colourless
(d10) [Cd(NH3)4]2+ – colourless
• Ammonium salts give ammonia gas with solutions of alkali(NaOH, KOH, Ca(OH)2 etc).
eg. NH4Cl(s) + NaOH(aq) → NH3(g) + Na+(aq) + Cl–(aq) + H2O(l)
The evolved NH3 gas can be tested with Nessler’s reagent or moist red litmus paper.
NH3(g) + Nerssler’s reagent → brown precipitate/colouration
• Qualitative analysis of a mixture of cations involves the separation of them to five groups. The scheme of qualitative analysis is based on the principle of selective precipitation. The precipitation of cations from a solution one at a time is called selective precipitation.
Group I
• Cold, excess dilute HCl is added to a solution containing a mixture of cations. Only Ag+, Pb2+ and Hg2 2+ will be precipitated as insoluble chlorides (AgCl, PbCl2, Hg2Cl2).
Group II
• After the separation of the insoluble chlorides in Group I, the filtrate is still acidic. When H2S is passed through the solution, only insoluble sulphides get precipitated.
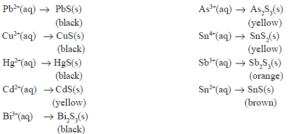 • The concentration of sulphide ion is relatively low because of the higher concentration of H+ ions. Other cations such as Mn2+ , Zn2+ , Ni2+ and Co2+ with higher Ksp values of their respective sulphides will remain in the solution.
• The concentration of sulphide ion is relatively low because of the higher concentration of H+ ions. Other cations such as Mn2+ , Zn2+ , Ni2+ and Co2+ with higher Ksp values of their respective sulphides will remain in the solution.
Group III
• The filtrate from Group II is boiled for a few minutes to expel all the dissolved H2S. Then boil the filtrate for a few minutes with conc.HNO3 to oxidise Fe2+ to Fe3+ . The solution is treated with NH4Cl and NH4OH.
Fe3+ (aq) → Fe(OH)3(s) (reddish brown)
Al3+ (aq) → Al(OH)3(s) (white gelatinous)
Cr3+ (aq) → Cr(OH)3(s) (green)
Group IV
• The filtrate from Group III contains OH– ions and is basic. H2S is passed through this solution in the presence of OH– ions. Then H+ ions produced from H2S are neutralised by hydroxyl ions.
• The above equilibrium shifts to the right and the concentration of S2- ions increases
Group V
• Boils off H2S from Group IV filtrate and add a little amount of NH4Cl and NH4OH in excess. Heat the solution, then add (NH4)2CO3 solution. Here Ca2+, Sr2+ and Ba2+ ions are precipitated as carbonates.

Aren’t there any questions in the Qbank???🤷🏽♂️