• Nitrogen and hydrogen gases are used as raw materials.
• Nitrogen is obtained by the fractional distillation of liquid air.
• Hydrogen is obtained from naptha or natural gas as follows.
C6H14(g) + 6H2O(g) → 6CO(g) + 13H2(g)
in naptha
CH4(g) + H2O(g) → CO(g) + 3H2(g)
in natural gas
or partial oxidation with oxygen:
C6H14(g) + 3O2(g) → 6CO(g) + 7H2(g)
in naptha
2CH4(g) + O2(g) → 2CO(g) + 4H2(g)
in natural gas
• Nitrogen and hydrogen form an equilibrium mixture containing ammonia.
N2(g) + 3H2(g) ⇌ 2NH3(g) ΔH = -92 kJ mol-1
• Le Chatelier’s principle suggests that increase in pressure and decrease in temperature will increase the proportion of ammonia at equilibrium.
• High pressure obviously gives a high yield of ammonia, but the higher the pressure greater the cost and maintenance of equipment. The favoured pressure nowadays is 250 atm.
• The temperature must be low to give a higher yield of ammonia. But at low temperature the rate of reaction is so low that it makes the process uneconomical. In practice, the optimum temperature is usually about 450 0C. As the reaction is exothermic the system must be cooled.
• In addition to pressure and temperature, the catalyst is a vitally important variable. Here iron is used as a catalyst and small amounts of potassium oxide and aluminium oxide are used as promoters.
• Low concentrations of NH3 is favourable for the forward reaction. So NH3 is cooled under pressure and the liquid ammonia is removed.

• Production of nitric acid, fertilizers and nylon
• Petroleum industry utilizes ammonia in neutralizing the acid constituents of crude oil.
• Used in water and waste water treatment, such as pH control, in solution form to regenerate weak anion exchange resins.
• Used in stack emission control systems to neutralize sulphur oxides from combustion of sulphur-containing fuels.
• Used as a refrigerant in industrial refrigeration systems found in the food, beverage, petrochemical and cold storage industries.
• Used in the rubber industry for the stabilization of natural and synthetic latex to prevent premature coagulation.
• Ammonia and carbon dioxide are used as raw materials.
• Production of urea is a two step process.
(i) 2NH3(g) + CO2(g) ⇌ NH2COONH4(s)
(ii) NH2COONH4(s) ⇌ CO(NH2)2(aq) + H2O(l)
• Reaction of the step 1 is fast and exothermic and essentially goes to completion under reaction conditions used industrially. Unreacted NH3 and CO2 are fed into the first step.
• Reaction of step 2 is slower and endothermic and does not go to completion. The conversion is in the order of 50-80%.
• Urea is a popular solid nitrogen fertilizer because of its high nitrogen content (46%).
• Urea is used in the manufacture of urea-formaldehyde polymer.
• Ammonia, air and water are used as raw materials.
• The oxidation of ammonia by air to give nitric oxide is an exothermic reaction. The temperature is adjusted to and maintained at about 900 °C by controlling the flow rate of the gases.
• The process is operated under increased pressure because this packs more reactants into the same capacity plant and increases the rate slightly by increasing the number of molecular collisions per second at the catalyst surface.
• An excess air is used to ensure complete oxidation of NH3
• Cold air is added to the mixture as it leaves the catalyst because the next stage is an exothermic equilibrium and is therefore favoured by low temperature.
• Extensive cooling of the gases is necessary before nitrogen dioxide is absorbed in water in the presence of air to give nitric acid.
Thus the actual conditions used leading to about 96% conversion are
• Pressure: 1 – 9 atm
• Temperature : 850 -1225 0C
• Catalyst : platinum containing 10% rhodium
• Used in the synthesis of ammonium nitrate for use as a fertilizer and in explosives.
• Used to prepare nitrates which are of great importance in industry.
• NaNO3 is used as a preservative for meat.
• KNO3 is used in fertilizers and for making gun powder.
• AgNO3 is used to prepare photographic film and paper.
• For the preparation of aquaregia.
• Used to clean soldering surfaces
• Phosphorus is an essential nutrient for all living organisms.
• An important phosphorus containing fertilizer for plants is superphosphate which is a mixture of calcium dihydrogenphosphate Ca(H2PO4)2 and hydrated calcium sulphate (gypsum) CaSO4.2H2O.
• Eppawela apatite [3Ca3(PO4)2.CaX2 or Ca5(PO4)3X where X = F/Cl/OH] is a good raw material for the prodution of phosphate fertilzers.
• Apatite is insoluble and made soluble for short term crops by complete and partial acidulation.
• Sulphuric acid, nitric acid, hydrochloric acid and phosphoric acid can be used for acidulation.
3Ca3(PO4)2.CaX2(s) + 7H2SO4(aq) →3Ca(H2PO4sub>2)2(s) + 7CaSO4(s)+ 2HX(aq) —(1)
3Ca3(PO4)2.CaX2(s) + 14HCl(aq) → 3Ca(H2PO4)2(s) + 7CaCl2(s)+ 2HX(aq) —–(2)
• Apatite is finely ground and mixed with acid and left for 4-6 weeks. Then the product
single superphosphate (SSP) is obtained.
• Addition of ammonium sulphate to products of reaction (2) produces a non hygroscopic fertilizer.
CaCl2(s) + (NH4)2SO4(aq) → CaSO4(s) + 2NH4Cl(aq)
• Sulphur or sulphur containing minerals, air and water are used as raw materials. Sulphur dioxide produced during the extraction of metals such as lead and zinc from their sulphide ores can also be used.
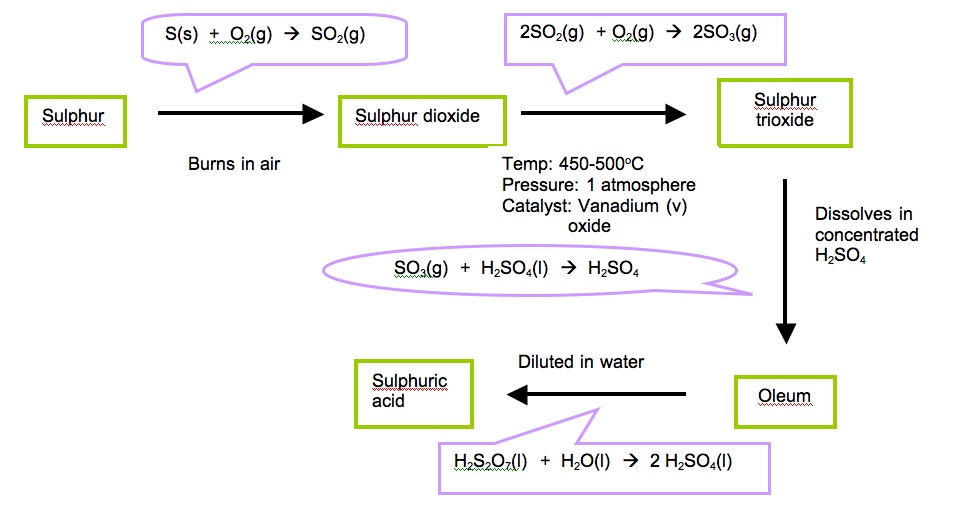
• The reaction between sulphur dioxide and oxygen is reversible. Sulphur trioxide continuously breaks down again to sulphur dioxide and oxygen. So the mixture is passed over several beds of catalyst to let the gases react again.
• The sulphur trioxide is removed between the last two beds of catalyst in order to increase the yield.
• As the reaction of formation of sulphur trioxide is exothermic and three moles of reactants form two moles of products, Le Chatelier’s principle predicts that the maximum yield of SO3 at equilibrium will be obtained at high pressure and at low temperature.
• In practice, a compromise temperature of 450 °C is chosen. This is the lowest that can be used without reducing the reaction rate to an unacceptable level. There are other reasons for keeping the temperature as low as possible. Fuel cost and corrosion of reaction chambers increase rapidly with rising temperature.
• At 450 °C conversion to SO3 is 97% and this high conversion even at atmospheric pressure, makes it unnecessary to carry out the process at increased pressure.
• As the reaction proceeds, the heat evolved in the exothermic reaction moves the systemto higher temperature. At this higher temperature the percentage conversion to SO3 ismuch reduced. Thus, it is necessary to cool gases between successive beds of catalyst.This is done using cold water pipes. The water is converted to steam and used to generate power(electricity).
• The sulphur trioxide is dissolved in concentrated acid rather than water. If it is dissolved in water, a thick mist of acid forms. This would be a pollution hazard.
• Oleum is mixed carefully with water to produce concentrated sulphuric acid.
• Manufacture of phosphate fertilizers
• Manufacture of ammonium sulphate fertilizers
• Manufacture of synthetic fibres rayon and plastics
• In the production of detergents – mostly alkyl and aryl sulphonates
• In the production of dyes, explosives and drugs
• In the production of battery acid
• Drying gases (Cl2)
