Aluminum (also called Aluminium) is the third most abundant element in the earth’s crust. It is commonly used in the household as aluminum foil, in crafts such as dyeing and pottery, and also in construction to make alloys. In its purest form the metal is bluish-white and very ductile. It is an excellent conductor of heat and electricity and finds use in some wiring. When pure it is too soft for construction purposes but addition of small amounts of silicon and iron hardens it significantly.
The electron configuration for Aluminum is 1s22s22p63s23p1.
Aluminum has three oxidation states. The most common one is +3. The other two are +1 and +2.
Reaction of aluminium with air
Aluminium is a silvery white metal. The surface of aluminium metal is covered with a thin layer of oxide that helps protect the metal from attack by air. So, normally, aulumium metal does not react with air. If the oxide layer is damaged, the aluminium metal is exposed to attack. Aluminium will burn in oxygen with a brilliant white flame to form the trioxide alumnium(III) oxide, Al2O3.
4Al(s) + 3O2(l) → 2Al2O3(s)
Reaction of aluminium with water
Aluminium is a silvery white metal. The surface of aluminium metal is covered with a thin layer of oxide that helps protect the metal from attack by air. So, normally, aulumium metal does not react with air. If the oxide layer is damaged, the aluminium metal is exposed to attack, even by water.
Reaction of aluminium with the halogens
Aluminium metal reacts vigorously with all the halogens to form aluminium halides. So, it reacts with chlorine, Cl2, bromine, I2, and iodine, I2, to form respectively aluminium(III) chloride, AlCl3, aluminium(III) bromide, AlBr3, and aluminium(III) iodide, AlI3.
2Al(s) + 3Cl2(l) → 2AlCl3(s) → Al2Cl6(s)
AlCl3 is an electron defficient compound and also highly covalent when anhydrous. AlCl3 exists as a dimer thus attaining an octet of electrons.
2Al(s) + 3Br2(l) → Al2Br6(s)
2Al(s) + 3I2(l) → Al2I6(s)
Reaction of aluminium with acids
Aluminium metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated Al(III) ion together with hydrogen gas, H2. The corresponding reactions with dilute hydrochloric acid also give the aquated Al(III) ion. Concentrated nitric acid passivates aluminium metal.
2Al(s) + 3H2SO4(aq) → 2Al3+(aq) + 2SO42-(aq) + 3H2(g)
2Al(s) + 6HCl(aq) → 2Al3+(aq) + 6Cl–(aq) + 3H2(g)
Reaction of aluminium with bases
Aluminium dissolves in sodium hydroxide with the evolution of hydrogen gas, H2, and the formation of aluminates of the type [Al(OH)4]–.
2Al(s) + 2NaOH(aq) + 6H2O → 2Na+(aq) + 2[Al(OH)4]– + 3H2(g)
Carbon is a Group 14 element and is distributed very widely in nature. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. Carbon is present as carbon dioxide in the atmosphere and dissolved in all natural waters. It is a component of rocks as carbonates of calcium (limestone), magnesium, and iron.
Coal, petroleum, and natural gas are chiefly hydrocarbons. Carbon is unique among the elements in the vast number of variety of compounds it can form.
Carbon is found free in nature in three allotropic forms: amorphous, graphite, and diamond. Graphite is one of the softest known materials while diamond is one of the hardest
More recently, another form of carbon, buckminsterfullerene, C60, was discovered. This form of carbon is the subject of great interest in research laboratories today.
Pure carbon is available in a number of different forms (allotropes). The most common form of pure carbon is α-graphite. This is also the thermodynamically most stable form. Diamond is a second form of carbon but is much less common. Other forms of carbon include the fullerenes. Whereas diamond and graphite are infinite lattices, fullerenes such as buckminsterfullerene, C60, is a discrete molecular species. Amorphous forms of carbon such as soot and lampblack are materials consisting of very small particles of graphite.

Atom arrangements in the most common allotrops of carbon: α-graphite.
As diamond has a slightly more compact structure its density is greater than that of graphite. The appearance of diamond is well known and it is also one of the hardest materials known. Like graphite, it is relatively unreactive but does burn in air at 600-800°C. Each carbon atom is bound to four neighbours at a distance of 154.45 pm in a tetrahedral fashion and so each diamond crystal is a single giant lattice structure. In principle (and in practice!) graphite may be converted into diamond by the application of heat and pressure.

Crystal strucutres of diamond.
Recently another allotrope of carbon was characterized. Whereas diamond and graphite are infinite lattices, buckminsterfullerene, C60, is a discrete molecular species. The buckminsterfullerene molecule is a net of 12 pentagons and 20 hexagons folded into a sphere. The effect is very similar to the patchwork of 12 pentagonal and 20 hexagonal pieces of leather that sewn together make up an association football (soccer ball). The name buckminsterfullerene (or buckyball was coined because of the relationship between the structure of C60 and R. Buckminster Fuller’s geodesic dome designs. Buckminsterfullerene is now commercially available and has also been identified in interstellar space and soot.
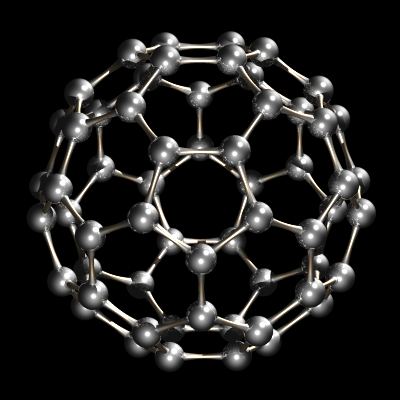
C60, Buckminsterfullerene.
Nanotubes are related to fullerenes. They are tubes giving the appearance of rolled graphite, although they are made from graphite. They are open ended while fullerenes are closed structures.
Oxides of carbon
CO is a colourless, neutral, and poisonous gas. CO is used as an industrial fuel. CO is a Lewis acid.
CO2 is a colourless acidic gas and a non polar molecule. Solid CO2 (dry ice) contains dispersion interactions. Dry ice is used as a freezing agent in food industry, in causing artificial rain, etc.
Carbonic acid
Carbonic acid is weak diprotic acid. There are two salts derived from H2CO3 .
CO2 + H20 → H2CO3
H2CO3 → H+ + HCO3–
HCO3– → H+ + CO32-
Reaction of carbon with air
Carbon, as graphite, burns to form gaseous carbon (IV) oxide (carbon dioxide), CO2. Diamond is a form of carbon and also burns in air when heated to 600-800°C – an expensive way to make carbon dioxide!
C(s) + O2(g) → CO2(g)
When the air or oxygen supply is restricted, incomplete combustion to carbon monoxide, CO, occurs.
2C(s) + O2(g) → 2CO(g)
This reaction is important. In industry, air is blown through hot coke. The resulting gas is called producer gas and is a mixture of carbon monoxide (25%), carbon dioxide (4%), nitrogen (70%), and traces of hydrogen (H2), methane (CH4), and oxygen (O2).
Reaction of carbon with water
Carbon, either as graphite or diamond does not react with water under normal conditions. Under more forsing conditions, the reaction becomes important. In industry, water is blown through hot coke. The resulting gas is called water gas and is a mixture of hydrogen (H2, 50%), carbon monoxide (CO, 40%), carbon dioxide (CO2, 5%), nitrogen and methane (N2 + CH4, 5%). It is an important feedstock gas for the chemical industry.
C + H2O → CO + H2
This reaction is endothermic (ΔH° = +131.3 kJ mol-1; ΔS° = +133.7 J K-1 mol-1) which means that the coke cools down during the reaction. To counteract this, the steam flow is replaced by air to reheat the coke allowing further reaction.
Reaction of carbon with the halogens
Graphite reacts with fluorine, F2, at high temperatures to make a mixture of carbon tetrafluoride, CF4, together with some C2F6 and C5F12.
C(s) + excess F2(g) → CF4(g) + C2F6 + C5F12
At room temperature, the reaction with fluorine is complex.
The other halogens appear to not react with graphite.
Carbon has a very high melting and boiling point and rapidly combines with oxygen at elevated temperatures. In small amounts it is an excellent hardener for iron, yielding the various steel alloys upon which so much of modern construction depends. An important (but rare) radioactive isotope of carbon, C-14, is used to date ancient objects of organic origin.
N2 is a colourless gas. It contains a triple bond, , with a short bond length of 1.09 °A. It has high dissociation bond energy 946 kJ mol-1 and it is an inert gas.
• Liquid N2 (boiling point -196 °C) is used as a coolant. N2 gas is used in the manufacture of ammonia.
• Nitrogen shows variable oxidation states.
-3 -2 -1 0 +1 + 2 +3 +4 +5
NH3 N2H4 NH2OH N2 N2O NO N2O3 N2O4 N2O5
Oxo acids of nitrogen
Nitrous acid (HNO2 ) is unstable except in dilute solutions. HNO2 is formed when acid reacts with metal nitrites.
NaNO2(s) + HCl(aq) → NaCl(aq) + HNO2(aq)
Nitric acid (ΗΝΟ3 ) is a colourless liquid which boils at 86 °C. ΗΝΟ3 a stable, strong acid as well as a strong oxidizing agent. Concentrated ΗΝO3 acid is usually yellow. In the light, it decomposes to form nitrogen dioxide and oxygen.
4HNO3(l) → 4NO2(g) + O2(g) + 2H2O(l)
Compounds
The two most common compounds of nitrogen are Potassium Nitrate (KNO3) and Sodium Nitrate (NaNO3). These two compounds are formed by decomposing organic matter that has potassium or sodium present and are often found in fertilizers and byproducts of industrial waste.
Nitrides are compounds of nitrogen with a less electronegative atom; in other words it’s a compound with atoms that have a less full valence shell. These compounds form with lithium and Group 2 metals. Nitrides usually has an oxidation state of -3
3Mg+ N2→Mg3N2
Nitrogen goes through fixation by reaction with hydrogen gas over a catalyst. This process is used to produce ammonia. As mentioned earlier, this process allows us to use nitrogen as a fertilizer because it breaks down the strong triple bond held by N2. The famous Haber-Bosch process for synthesis of ammonia looks like this:
N2+3H2→2NH3
Ammonia is a base and is also used in typical acid-base reactions.
ΝH3 acts as an oxidizing agent and as well as an acid
2Na(s) + 2NH3(l) → 2NaNH2(s) + H2(g)
ΝH3 acts as a weak reducing agent.
3NH3(l) + 2Cl2(g) → 2 N2(g) + 6HCl(g)
3CuO(s) + 2NH3(l) → N2(g) + 3Cu(s) + 3H2O(l)
ΝH3 acts as a weak reducing agent.
2NH3(aq)+H2SO4→(NH4)2SO4(aq)
Ammonium salts
Ammonium salts decompose quite readily on heating.
(NH4)2CO3(s) →Δ 2NH3(g) + CO2(g) + H2O(l)
NH4Cl(s) →Δ NH3(g) + HCl(g)
NH4NO2(s) →Δ N2(g) + 2H2O(l)
NH4NO3(s) →Δ N2O(g) + 2H2O(l)
(NH4)2Cr2O7(s) →Δ N2(g) + Cr2O3(s) + 4H2O(s)
Nitrides use a variety of different oxidation numbers from +1 to +5 to for oxide compounds. Almost all the oxides that form are gasses, and exist at 25 degrees Celsius. Oxides of nitrogen are acidic and easily attach protons.
N2O5+H2O→2HNO3(aq)
The oxides play a large role in living organisms. They can be useful, yet dangerous.
Hydrides of nitrogen include ammonia (NH3) and hyrdrazine (N2H4).
Allotropes of Oxygen
There are two allotropes of oxygen; dioxygen (O2) and trioxygen (O3) which is called ozone. The reaction of converting dioxygen into ozone is very endothermic causing it to occur rarely and only in the low atmosphere. The reaction is shown below:
3O2(g)→2O3(g)
Ozone is unstable and quickly decomposes back to oxygen but is a great oxidizing agent
Group 17 elements (halogens) fluorine, chlorine, bromine, and iodine react with oxygen to form oxides. Fluorine forms two oxides with oxygen which are F2O and F2O2. Both fluorine oxides are called oxygen fluorides because fluorine is the more electronegative element. One of the fluorine reactions is shown below.
O2(g)+F2(g)→F2O2(g)
Rhombic Sulphur (Octahedral or Alpha Sulphur)
Rhombic sulphur is prepared by dissolving roll sulphur in carbon disulphide, and then evaporating the solution slowly, at room temperature.Rhombic or octahedral sulphur consists of rings of 8 atoms of sulphur. It is the most stable of all the allotropes of sulphur. It is soluble in carbon disulphide, benzene, chloroform, etc., but is insoluble in water. It is non-conductor of heat and electricity. It is transparent and pale yellow in color.
Monoclinic Sulphur (Prismatic or Beta Sulphur)
This allotrope of sulphur is prepared by melting roll sulphur in a dish. The molten sulphur is allowed to cool slowly. The top layer solidifies first and forms a crust. Two holes are made in the crust with the help of a heated nail. The molten sulphur is poured out through one of the holes. Then with the help of a knife the crust is carefully peeled off
Pale-yellow, transparent needle shaped crystals are seen projecting out form the inner surface of the dish. These are the crystals of monoclinic sulphur. Monoclinic sulphur also consists of 8 atom rings. It is stable only above 96oC. When it cools down below 96oC, it changes to rhombic sulphur i.e., 96oC is the transition temperature of this sulphur.
Plastic Sulphur
On heating sulphur, till almost the boiling point and suddenly cooling it by pouring into cold water a viscous mass is formed. This sudden cooling does not allow sufficient time to the molecules to rearrange themselves to form monoclinic or rhombic forms of sulphur. Hence the molecules form an interwined mass, consisting of both rhombic and monoclinic varieties of sulphur. This is called plastic sulphur
This type of sulphur is a dark brown or even black, sticky substance. It is elastic. It has no sharp melting point. It does not dissolve in carbon disulphide. On standing, it slowly changes to the rhombic forms, as it gains the eight atom ring structure.
Colloidal Sulphur
This type of sulphur is prepared by passing hydrogen sulphide through a cooled saturated solution of sulphur dioxide in water, or by adding a solution of sulphur and alcohol in water. Colloidal sulphur is soluble in carbon disulphide. It is used in medicine.
Magnesium burns in sulphur di oxide giving magnesium oxide and sulphur.


Potassium reacts with sulphur dioxide forming potassium sulphite and potassium thiosulphate.


Sulphur dioxide reacts with alkali solution to give salt and water. [sulphites and bisulphites] When sulphur di oxide is bubbled through sodium hydroxide solution no precipitate is formed since sodium sulphite is a soluble salt.When sulphur dioxide is passed through Ca (OH)2 solution, a white precipitate of insoluble calcium sulphite is formed. If excess sulphur dioxide is passed, the precipitate disappears forming soluble calcium bi sulphite.











Oxidises magnisium to magnisium ion(Mg+2)
 Oxidises hydrogen silphide to sulphur
Oxidises hydrogen silphide to sulphur

In the presence of moisture SO2 liberated nascent hydrogen and reduction takes place by addition of hydrogen.
Example:


Potassium dichromate, potassium permanganate and nitric acid are reduced by the action of SO2 by removal of oxygen.Example:


 Sulphur dioxide gas exhibits bleaching properties in presence of moisture. It dissolves in water liberating nascent hydrogen. Coloring matter is bleached by reaction with nascent hydrogen. Nascent hydrogen removes oxygen atoms from the coloring matter (reduces coloring matter) and it loses its color. This bleaching is temporary because the bleached product on exposure to atmospheric oxygen adds on oxygen atoms from air and regains its original color.
Sulphur dioxide gas exhibits bleaching properties in presence of moisture. It dissolves in water liberating nascent hydrogen. Coloring matter is bleached by reaction with nascent hydrogen. Nascent hydrogen removes oxygen atoms from the coloring matter (reduces coloring matter) and it loses its color. This bleaching is temporary because the bleached product on exposure to atmospheric oxygen adds on oxygen atoms from air and regains its original color.
Uses of sulphur dioxide
The various uses of sulphur dioxide are:
1) In the manufacturing of sulphuric acid, sulphites, and hydrogen sulphite.
2) In the sugar industry for refining and decolorizing sugar.3) For refining kerosene, and other petroleum products.
4) As a disinfectant.5) As a fumigant.
6) For bleaching delicate articles.7) As antichlor, to remove the excess chlorine from substances that have been bleached by chlorine.
8) As a solvent for glue.9) As a refrigerant in household refrigerators.
10) As a preservative for wines, meat, dry fruits etc.
Sulphuric acid, H2SO4 is a colorless, odorless, extremely corrosive, oily liquid. It was initially called oil of vitriol.
Uses of sulphuric acid
This all means that oxidising ability falls as you go down the Group.
Position of noble gas in periodic table
Noble gases are also known as inert gases and do not take part in chemical reactions. They have their outermost shell complete and thus remain stable. They do not generally combine with other substances, nor are they affected by oxidising agents or by reducing agents. They are placed in the 18 or VIIIA group. Since, the outermost shell is complete, the valency is zero, hence VIIIA group is also referred to as zero group.
occurrence of noble gas
Noble gases always occur in free state because of their inert nature. All the noble gases, except radon are present in air in small amounts. The relative abundance of the noble gases in air is = 1%. Helium is present in natural gas up to the extent of 10 per cent. It is also present in small quantities in the minerals of radioactive elements. Water from certain springs also gives off gases, which are rich in helium and argon.
Uses of noble gas
Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes (arc welding of metals or alloys) and for filling electric bulbs. It is also used in the laboratory for handling substances that are air-sensitive.
Helium is a non-flammable and light gas. Hence, it is used in filling balloons for meteorological observations. It is also used in gas-cooled nuclear reactors. Liquid helium (boiling point 4.2K) finds use as cryogenic agent for carrying out various experiments at low temperatures. It is used to produce and sustain powerful super conducting magnets, which form essential part of modern NMR spectrometers and Magnetic Resonance Imaging (MRI) systems for clinical diagnosis.
Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. There are no significant uses of xenon and krypton. They are used in light bulbs designed for special purposes.
