• Capital • Technology
• Availability of raw materials • Power (electricity, fuel, etc.)
• Labour • Transport facilities and the market
• Waste management • Controlling the environmental pollution
• Government rules and regulations
• Characteristics of natural resources that can be used as raw materials for an industry
• Occurring as large ores appropriate for long term usage
• Easy access • High purity
• s- block elements do not occur freely in the nature because they are very reactive and they exist as compounds
Rock salt – NaCl
Sea water – NaCl, MgCl2, CaCl2, CaSO4, Ca(HCO3)2, MgSO4
Silvine – KCl
Borax – Na2B4O7.10H2O
Beryl – 3BeO.Al2O3.6SiO2
Magnesite – MgCO3
Dolomite – CaCO3.MgCO3
Limestone,Marble,Oyster shells – CaCO3
Gypsum – CaSO4.2H2O
Fluorspar – CaF2
Apatite – Ca5(PO4)3X or 3Ca3(PO4)2.CaX2 (X = F, Cl,OH)
• Sodium is extracted using electrolysis of molten NaCl. CaCl2 is added to reduce the
melting point of NaCl to 600 °C.
• At the cathode,
Na+(l) + e→ Na(l)
• At the anode,
2Cl–(l) → Cl2(g) + 2e
• Anode and cathode are separated by a circular disc (steel gauze diaphragm) to prevent the reaction between chlorine gas and sodium.
• A large current is passed through the cell, but at a low voltage.
• Sodium vapour lamps
• Molten sodium is used as a coolant in nuclear reactors.
• Solid sodium is used to dry organic solvents like ether and benzene.
• Used in organic synthesis
• Used to synthesise sodamide (NaNH2) which is a strong reducing agent.
The places where salt produced are referred to as saltterns. In Sri Lanka two main saltterns are placed in Puttalam and Hambantota.
Characteristic features of the places where saltterns are established
• Plane land by the sea or lagoon • Less rainfall
• Dry air, more sunlight • Water impervious clay sand
• Sea water is used as the raw material.
• Sea water is pumped into the first tank of the salttern. The sea water is evaporated by sunlight. When the concentration of sea water increases CaCO3 gets precipitated in the first tank of the salttern. CaCO3 precipitate is allowed to settle down.
• Remaining solution is transferred into the second tank of the salttern and evaporated by sunlight. When the concentration further increases CaSO4 gets precipitated.
• Remaining solution is transferred into the third tank of the salttern and evaporated. When the concentration further increases NaCl gets precipitated. NaCl is removed from the third tank of the salttern. This NaCl contains Ca2+, Mg2+ and SO42- as impurities.
• Pure NaCl is not hygroscopic. But NaCl having the impurities is hygroscopic. Sodium chloride collected from the third tank is stored outside for nearly six months. During this storage period NaCl is almost purified as Ca2+ and Mg2+ salts absorb water from air and become solution while NaCl remains as a solid.
• Iodized salt is produced by mixing with KIO3.
• Cooking
• Food preservative (Maldivian fish, Pickle)
• Manufacture of Na metal, Na2CO3, NaHCO3 and NaOH
• Saline
• To reduce the melting point of ice
• As Mg2+ and Br- concentrations are high in bittern soultion it can be used to produce Mg and Br2.
• Sodium hydroxide is commercially produced by the electrolysis of aqueous sodium chloride in chloro-alkali cells.
• There are three types of chloro alkali cells
• Mercury cell • Diaphragm cell • Membrane cell
• The membrane cell is very similar to the diaphragm cell and the same reactions occur. The main difference is that two electrodes of membrane cell are separated by an ion selective membrane rather than by a diaphragm.
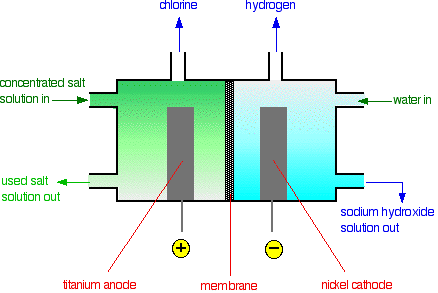
• The advantage of using membrane cell is that the NaOH that is produced is very pure and also uses less electricity, lowest environmental impact.
• The half reactions are
2Cl–(aq) → Cl2(g) + 2e (at anode)
2H2O(l) + 2e → 2OH–(aq) + H2(g) (at cathode)
• The overal reaction is
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
• The anode is made of titanium and cathode is made of nickel.
• The anodic and cathodic compartments are separated by a polymer cation-exchange membrane.
• The membrane can exchange cations and hence permits Na+ ions to migrate from anodic compartment to cathodic compartment.
• The flow of cations maintain electro-neutrality in the two compartments because, during electrolysis, charge is removed at the anode and supplied at the cathode.
• OH– would react with Cl2, and spoil the process. But migration of OH– is suppressed because the membrane does not exchange anions.
• NaOH solution is partially evaporated and allowed to cool.
• Chlorine is produced as a by-product.
• Use in the laboratory as a convenient strong base
• Absorb carbon dioxide and other acidic gases
• Manufacture of soap, paper, atificial silk and dye stuffs
• Treatment of effluents for removal of heavy metals (as hydroxides) and of acidity
• Used directly or in combination form as a bleach for textiles, wood, and paper pulps.
• Disinfection of drinking water
• One quarter of the hydrochloric acid produced in U.K. is synthesized from chlorine and hydrogen.
• Used in recovery of tin, titanium and magnesium from scrap.
• Used to produce chlorinated rubbers, insecticides, dyes and drugs.
• Used in the manufacture of polymeric materials such as polyvinyl chloride
Oils, fats or their fatty acids and inorganic water soluble bases (NaOH, KOH) are used as raw materials.
• The industrial soap making involves four basic steps.
• Step 1 – Saponification
The saponification process involves the mixing of tallow (animal fat), coconut oil or vegetable oil with sodium hydroxide and the application of heat. The process results in formation of soap, which is a salt of long chain carboxylic acid.

• Step 2 – Removal of glycerin
Glycerin is more valuable than soap, and hence most of it is removed for its uses in more expensive cosmetic products. Some of the glycerin is left in soap to make it soft and smooth.
• Step 3 – Soap purification
In the soap purification stage any remaining sodium hydroxide is neutralized with a weak acid like citric acid and two thirds of the remaining water is removed to obtain pure soap.
• Step 4 – Finishing
The final stage of industrial soap manufacturing process, finishing stage involves mixing of additives such as colours, preservatives and perfume into soap, which is then shapedinto bars for sale.
• Instead of NaOH, KOH could be used. The soap manufactured using KOH is softer on skin. Therefore KOH is used mainly in the manufacture of baby soap.
• The percentage of RCOO–Na+ in the soap is referred as total fatty matter(TFM) value.
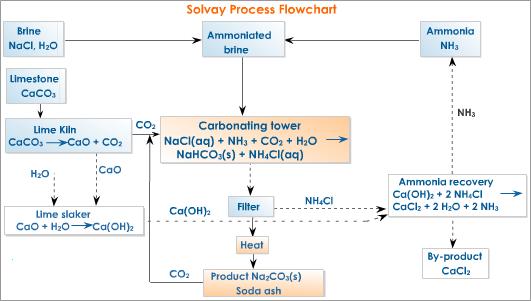
• Brine (concentrated NaCl solution), limestone and ammonia (manufactured by Haber process) are used as raw materials.
• NH3 gas is dissolved in brine. This reaction is exothermic. Therefore low temperatures are favoured.
• Counter current principle is used to achieve higher efficiency of dissolving.
• Brine saturated with NH3 is allowed to react with CO2(g) which is obtained by heating limestone. The reaction is exothermic. Therefore, low temperatures are favoured.
• Again the counter current principle is used for achieving higher efficiency.
Here, following reversible reactions take place.
NH3(aq) + H2O(l) → NH4+ (aq) + OH–(aq)
OH– (aq) + CO2 (aq) → HCO3–(aq)
• As OH– is removed by the second reaction more and more OH– ions are formed by the first reaction.
• When HCO3– concentration increases NaHCO3 crystallizes.
Na+(aq) + HCO3–(aq) → NaHCO3(s)
• Low temperature is maintained to facilitate the separation of solid NaHCO3
• NaHCO3(s) is isolated and heated. CO2 formed is used again.
2NaHCO3(s) →Δ Na2CO3(s) + CO2(g) + H2O(g)
• Net reaction for the formation of NaHCO3 is
NaCl (aq) + NH3(aq) + CO2(aq) + H2O(l) → NaHCO3(s) + NH4Cl(aq)
• The NH4Cl and lime are used to regenerate NH3. This NH3 is used again.
CaO(s) + 2NH4Cl(aq) → CaCl2(aq) + 2NH3(aq) + H2O(l) or
Ca(OH)2(s) + NH4Cl(aq) → CaCl2(aq) + NH3(aq) + H2O(l)
• Washing soda • Manufacture of glass
• Softening of hard water • Manufacture of detergents
• Manufacture of soap • Manufacture of paper
• The solubility of KHCO3 is greater than that of NaHCO3 and cannot be precipitated by the above method. Therefore, K2CO3 could not be manufactured using Solvay process.
• In this process alternate layers of crushed CaCO3 and fuel (firewood) are fed into the kiln from top. A fire is started at the bottom and gradually spreads upward.
• The high temperature causes CO2 to be expelled from the kiln leaving CaO. After the kiln is cooled the quicklime is withdrawn from the bottom.
• Pollution due to the heat released to the environment.
• Air pollution caused by CO2 released and fine particles emitted.
CaCO3(s) ![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
Dissociation temperature of CaCO3 (900 °C) is relatively high, if the fuel (firewood) does not supply this temperature CaCO3 may not fully dissociate.
• If CO2 is not expelled fully from the kiln, it can combine with CaO again and form CaCO3 (as the reaction is reversible).
• CaO gets mixed with ash of the burnt fuel.
• Production of slaked lime and milk of lime
• Manufacture of calcium carbide
• Reducing acidity of the soil
• Manufacture of bleaching powder
• Construction of building
• Absorbing acidic gases
• Heating limestone to obtain quicklime (CaO)
• Sprinkling water on CaO to obtain slaked lime [Ca(OH)2(s)] and allow to cool
• Passing Cl2(g) over wet solid Ca(OH)2 at room temperature for about 12-15 hours in rotating kilns with intermittent raking
• Counter current principle is used for higher efficiency of the reaction.
CaCO3(s)![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
CaO(s) + H2O(l) → Ca(OH)2(s)
3Ca(OH)2(s) + 2Cl2(g) →Ca(OCl)2.Ca(OH)2.CaCl2.2H2O(s)
• Bleaching agent
• Disinfectant (especially for water)
• Quicklime (CaO) and coke (C) are heated in an electric arc at a temperature of
about 2000 °C.
CaO(s) + 3C(s) → CaC2(s) + CO(g)
2CaO(s) + 5C(s) → 2CaC2(s) + CO2(g)
• CaC2 reacts with H2O and produces C2H2.
CaC2(s) + H2O(l) → Ca(OH)2(s) + C2H2(g)
• In the production of oxyacetylene flame
• Used to induce flowering
• Used to induce ripening of fruits
