• The phenomenon of the existence of compounds with different atomic arrangements while having the same molecular formula is called isomerism.
• The isomers of a compound can show different physical and chemical properties from one another.
• The isomerism of organic compounds can be classified as follows.
Structural isomerism
Structural isomerism is the phenomenon of having compounds with different structural formulae (without considering the spatial orientation of bonds) for the same molecular formula.
Chain isomerism
Chain isomerism occurs when the nature of the carbon chain changes for the same molecular formula in the same homologous series

Position isomerism
Though there is the same molecular formula, the same functional group/substituent and the same carbon chain when there is a change in the carbon atom to which the functional group/substituent is attached or a change in the location of the active position, then there occurs position isomerism.
Functional group isomerism
Functional group isomerism is the existence of structures with different functional groups for the same molecular formula.

Stereoisomerism
Stereoisomerism is the existence of compounds whose structures differ from each other only in the orientation of bonds in three dimensional space (i.e. they have the same molecular formula and the same structural formula).
A pair of stereoisomers whose 3D – structures are mirror images of each other are enantiomers of each other. A pair of stereoisomers whose 3-D structures are not mirror images of each other are diastereomers of each other.
Diastereomerism
Geometric isomerism is one occasion where diastereomerism is seen. In a C=C double bond due to the π bond which exists in addition to the σ bond, these carbon atoms cannot freely rotate about the bond. It is possible to have different spatial arrengements of the groups joined to the two carbon atoms. These different arrangements which cannot be interconverted by rotation around carbon – carbon bond axis are known as geomentrical isomers. For geometrical isomers to exist, the two groups attached to each carbon atom of the double bond should not be identical
The words cis and trans are used to indicate the geometrical relationship of two groups attached to different carbon atoms in the same double bond. If the two groups are on the same side with reference to the plane which is perpendicular to the plane of the molecule going through the carbon – carbon axis of the double bond, then the relationship is cis. If the two groups are on opposite sides of the plane then the realtionship is trans.
For example

Enantiomerism
The isomers of which one is the mirror image of the other are known as enantiomers. A compound having a carbon atom which is joined to four different groups shows enatiomerism. Such a carbon atom is known as an asymmetric or chiral carbon atom
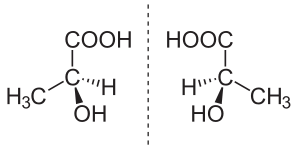
When plane – polarized light is passed through a solution containing only one enantiomer, the plane of polarization rotates. One enantiomer rotates the plane of polarization in one direction and the other enantiomer in the opposite direction. As the enantiomers rotate the plane of polarization, they are also known as optically active isomers
