Hund’s rule
Orbitals of the same energy (degenerate) are occupied by electrons singly so that their spins are parallel to make the maximum number of unpaired electrons and then doubly with their spins anti parallel.
Pauli’s exclusion principle
This principle states that no orbital can accommodate more than two electrons (In other words the set of quantum numbers for a certain electron of an atom is exclusive for it or no two electrons in an atom can have the same set of quantum numbers.)
Aufbau principle
It states that the filling up of electrons in the orbitals takes place according to the increasing order of energy of the orbitals in accordance with the Pauli exclusion principle.
Ascending order of energy in the sub energy levels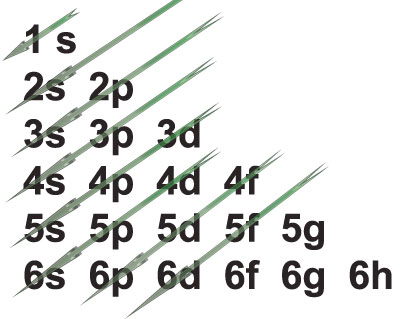
Graph showing the variation of first ionization energies of elements of atomic number
Anomalous behaviour seen from group II to III and from group V to VI is due to extra stability of half-filled (p³ ) and completely-filled shells (s² , p⁶ ). Electronic configurations d⁵ and d¹⁰ also exhibit extra stability.
