• It is necessary to indicate concentration for solutions (e.g. Fe2+(aq, 1.0 mol dm--3) and pressure in case of gases e.g. H2(g, 1.0 atm).
When a piece of active metal such as magnesium is dipped in a beaker of water, there will be some tendency for the metal atoms to leave elctrons and go into solution as metal ions.The electrons will be left behind on the metal. In a very short time, there will be a build -up of electrons on the metal, and it will be surrounded in the solution by a layer of positive ions.These will tend to stay close because they are attracted to the negative charge on the piece of metal. This is called double layer as shown in figure (a).
• Some of them will be attracted enough so that they will reclaim their electrons and stick back on to the piece of metal. A dynamic equilibrium will be established when the rate at which ions are leaving the surface is exactly equal to the rate at which they are joining it again. Here, the double layer reach to its equilibrium. At this point there will be a constant negative charge on the metal and a constant number of metal ions present in the solution around it. However, at the dynamic equilibrium, ions are continually leaving and rejoining the surface with equal rates. The potential that is created between the metal and adjecent layers of solution is known as the electrode potential.
• If a less active metal such as copper is considered, it forms its ions less readily. Any ions which do break away are more likely to reclaim their electrons and stick back on to the metal again. It will still reach an equilibrium position, but there will be a lesser charge on the metal, and fewer ions in the solution.
If both equilibria are considered together it is clear that the position of the magnesium equilibrium Mg2+(aq) + 2e ⇌ Mg(s) lies further to the left than that of the copper equilibrium Cu2+(aq) + 2e ⇌ Cu(s)
• By IUPAC convention, all these equilibria are written with the electrons on the left hand side of the equation. It is recommennded to stick on to this convention.
eg Calomel electrode
Hg2Cl2(s) + 2e ⇌ 2Hg(s) + 2Cl–
eg Silver-Silver chloride electrode
 AgCl(s) + e ⇌ Ag(s) + Cl–
AgCl(s) + e ⇌ Ag(s) + Cl–
eg. Hydrogen electrode
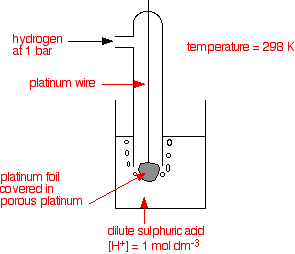 H+ + e ⇌ ½H2(g)
H+ + e ⇌ ½H2(g)
eg. Fe3+/Fe2+ electrode
Fe3+ + e ⇌ Fe2+
• The anode, or oxidation half-cell is always written on the left; the cathode, or reduction halfcell,is written on the right. The two electrodes are electrically connected by means of a salt bridge, denoted by two vertical bars.
• The cell terminals are at the extreme ends in the cell notation. Electrode and electrolyte interface of each half cell is separated by a single vertical bar. Other constituents are separated by commas.
Examples: Pt(s)|Fe2+(aq, 1 mol dm-3) , Fe3+(aq, 1 mol dm-3)
Hg(l),Hg2Cl2(s)|Cl(aq, 1 mol dm-3)
• When the half-reaction involves a gas, an inert material such as platinum serves as a terminal and as an electrode surface on which half-reaction occurs. The notation for the hydrogen electrode, written as a cathode and as an anode are given below respectively.
H+(aq, 1 mol dm-3)|H2(g)|Pt(s) || Pt(s)|H2(g)|H+(aq, 1 mol dm-3)
• Cell notations of several cells are given below.
Zn(s)|Zn2+(aq,1 mol dm-3 )||Cu2+(aq, 1 mol dm-3)|Cu(s)
Cd(s)|Cd2+(aq, 1 mol dm-3)||H+(aq, 1 mol dm-3)|H2(g)|Pt(s)
