• Aniline reacts with nitrous acid to give phenol.
Aromatic diazonium salts are more stable than aliphatic diazonium salts. Therefore, when this reaction is carried out at low temperatures, the conversion of the aromatic diazonium salt to the phenol can be slowed down, and the diazonium salt can be isolated.
With water
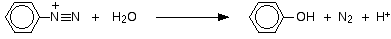
With CuCl,CuBr,KI and HBF4

With CuCN
C6H5N2+ + CuCN → C6H5CN + Cu+ + N2
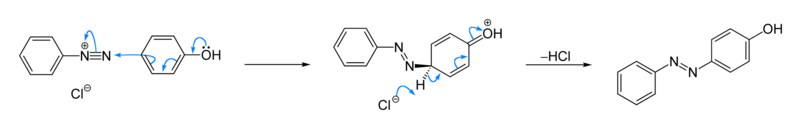
Benzene diazonuim chloride reacts with phenol to give an orange coloured compound, and with β -naphthol to give a red coloured compound.