• The cell in which the cell reaction cannot be reversed by providing electrical energy is called a “primary cell’.
eg-Daniel cell
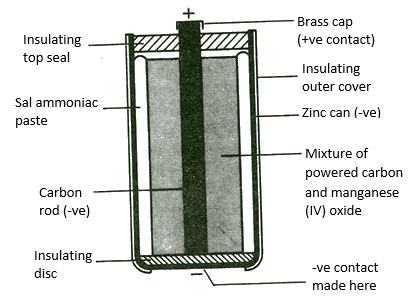
Electrolyte – NH4Cl/ZnCl2
(+) Pole – C/MnO2
(-) Pole – Zn
Reaction at (+) Pole(Cathode reaction) – 2NH4+(aq)+2MnO2(s)+2e → Mn2O3(s) + H2O(l)+2NH3(aq)
Reaction at (-) Pole(Anode reaction) – Zn(s) → Zn2+(aq) + 2e
Cell reaction – 2MnO2(s)+Zn(s)+H2O (l) → Zn2+(aq) + Mn2O3(s) +2OH–(aq)
Leclanche cell
Electrolyte -ZnSO4/CuSO4
(+) Pole – Cu
(-) Pole – Zn
Reaction at (+) Pole(Cathode reaction) – Cu2+(aq) + 2e → Cu(s)
Reaction at (-) Pole(Anode reaction) – Zn(s) → Zn2+(aq) + 2e
Cell reaction – Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
• A cell in which the reactants can be regenerated from the products by charging after
its discharge is referred to as a ‘secondary cell’.
eg. Lead accumulator

Electrolyte – dil. H2
SO4
Anode – Pb
Cathode – PbO2
Anode reaction during discharge – Pb(s) + SO42-(aq) → PbSO4(s) + 2e
Cathode reaction during discharge –
PbO2(s) + 4H+(aq) + SO42-(aq) + 2e → PbSO4(s) + 2H2O(l)
Electrochemcal cells in which reactants are continuously supplied from outside are referred as fuel cells. Dihydrogen and dioxygen, methane and dioxygen are commonly used for this purpose. One of the electrolyte used is concentrated KOH solution, maintained at 200 °C. Porous C-Ni electrodes are commonly used.
Relevent reactions for the dihydrogen-dioxygen fuel cell are given below.
Oxidation; 2H2(g) + 4OH–(aq) → 4H2O(l) + 4e
Reduction; O2(g) + 2H2O(aq) + 4e → 4OH–(aq)
Cell reaction 2H2(g) + O2(g) → 2H2O(l)
