• Under normal conditions benzene does not answer the tests for unsaturation. Therefore, benzene cannot have a structure similar to that of a simple alkene or an alkyne.
• Although the structure proposed for benzene by Kekule shows three double and three single bonds for the molecule, the bond length between any two adjacent carbon atoms in benzene is the same.
• The carbon – carbon bond length of benzene is 1.39 x 10-10 m which is in between the length of carbon – carbon double bond (1.34 x 10-10 m) and the length of carbon – carbon single bond (1.54 x 10-10 m).
• Therefore, the structure of benzene is considered to be a hybrid of the resonance structures given below.
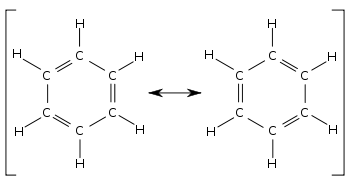
• The structure of benzene can be explained by molecular orbital theory. All its C atoms have undergone sp2 hybridization. Each carbon bears an unhybridized p orbital which can overlap with the unhybridized p orbitals on either sides of it.
• From this, a cyclic delocalized electron cloud is formed.
• Hence, the real structure of benzene is considered to be a hybrid of two Kekule structures.
• The real structure of benzene with delocalized electrons is more stable than the Kekule structure with three double bonds.
• The data for the standard enthalpy of hydrogenation helps to illustrate the stability of a benzene molecule.
The enthalpy change during this reaction is -120 kJ mol-1
The enthalpy change during this reaction is -208 kJ mol-1
But if benzene possesses three double bonds, its standard enthalpy of hydrogenation should be 3 x (-120 kJ mol-1
), that is -360 kJ mol-1
. Hence, benzene is more stable than its Kekule structures by an amount equal to (360-208) = 152 kJ mol-1
