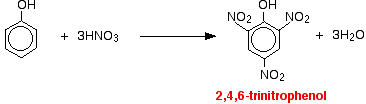Aromatic compounds, in which an -OH group is joined directly to a carbon atom of a benzene ring are called phenols. Alcohols and phenols dissociate in aqueous solutions as shown below.
ROH + H2O ⇌ RO– + H3O+
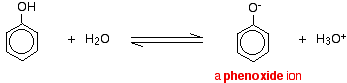
Phenols are more acidic than alcohols. This means that in the above equilibria, the equilibrium point for phenols is more towards the right than alcohols. The reason for this is that the stability of phenate ion relative to phenol is greater than the stability of the alkoxide ion relative to the alcohol. The phenate ion is more stable because its negative charge gets delocalized by resonance. In the alkoxide ion there is no such charge dispersion.
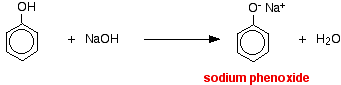
The higher acidity of phenols is confirmed by the following examples too. Although an alcohol reacts with sodium it does not react with NaOH. But phenol reacts with sodium as well as with NaOH. However, phenol is not acidic enough to react with Na2CO3.
2 C6H5OH + 2Na → 2C6H5O– Na++ H2
Unlike alcohols phenols do not undergo nucleophilic substitution reactions because,
(i) the C-O bond is shorter and stronger due to delocalization of lone pair of electrons
on the oxygen atom into the benzene ring. This can be shown by resonance.

(ii) phenyl cation is unstable.
• Due to the delocalization of the lone pairs of electrons which were on the oxygen atom with the benzene ring, the ring is rich with electrons. It has become very reactive towards electrophilic reagents. The O-H group of phenol is ortho, para directing. (It is not necessary to explain why the -OH group is ortho, para directing)
• When the electrophilic substitution reactions of phenol are compared with the corresponding reactions of benzene along with the relevant conditions, it is clear that the benzene ring of phenol, had become more reactive towards electrophiles.
Consider the following examples.
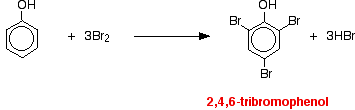
(i) Reacts immediately with bromine water to give a white precipitate of 2,4,6 – tribromophenol.
(ii) For nitration of phenol even dilute HNO3 is sufficiently reactive.
With dilute nitric acid
Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2 nitrophenol and 4-nitrophenol.
![]()
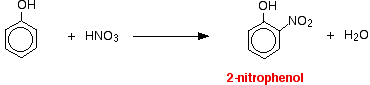
![]()
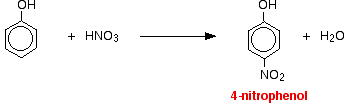
With concentrated nitric acid
With concentrated nitric acid, more nitro groups substitute around the ring to give 2,4,6-trinitrophenol
![]()