The Electromagnetic spectrum is divided into several regions based on different frequencies, wavelengths and their characteristics. The figure shown below shows the Electromagnetic Spectrum Diagram which consists of all the em waves with respect to the wavelength and frequencies.
The Regions of the Electromagnetic Spectrum are as follows:
Infrared wave:
The infrared wave lies between 300 GHz to 405 THz and hence the infrared wavelength is in between 750 nm – 1 mm. The near infrared lies between 0.75-1.4 μμm wavelength range of infrared region while the far infrared lies between 15 – 1000 μμm wavelength range of infrared region. Infrared spectrometers are generally used to study the Vibrational Spectra of molecules.
Visible light :
The frequencies in this region can be sensed by our eyes and interpreted as colors ranging from violet to red. With the violet having shorter wavelength and higher frequency while the red color have higher wavelength and shorter frequency.
Ultraviolet wave or rays :
The ultraviolet rays lie above the visible spectrum and are invisible to our eyes. These waves can be felt as sun burns.
X-rays :
The X-rays lie above the ultraviolet band and are produced by the sudden stoppage of the high speed charged particle by the use of metal target which absorbs these particles and hence the x-rays are emitted by such particles.
Gamma rays :
The Gamma rays are of extremely low wavelength and are produced by the radioactive decay of the radioactive atoms.
The term LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. The first laser was constructed in 1960.
(a) Action.
The action of a laser can be explained in terms of energy levels. A material whose atoms are excited emits radiation when electrons in higher energy levels return to lower levels. Normally this occurs randomly, i.e. spontaneous emission occurs, and the radiation is emitted in all directions and is incoherent. The emission of light from ordinary sources is due to this process. However, if a photon of exactly the correct energy approaches an excited atom, an electron in a higher energy level may be induced to fall to a lower level and emit another photon. The remarkable fact is that this photon has the same phase, frequency and direction of travel as the stimulating photon which is itself unaffected. This phenomenon was predicted by Einstein and is called stimulated emission
In a laser it is arranged that light emission by stimulated emission exceeds that by spontaneous emission. To achieve this it is necessary to have more electrons in an upper than a lower level. Such a condition, called an ‘inverted population’, is the reverse of the normal state to affairs but it is essential for light amplification, i.e. for a beam of light to increase in intensity as it passes through a material rather than to decrease as is usually the case.
One method of creating an inverted population is known as ‘optical pumping’ and consists of illuminating the laser material with light. Consider two levels of energies E1 and E2, where E2 > E1. If the pumping radiation contains photons of frequency (E2- E1)/h, electrons will be raised from level 1 to level 2 by photon absorption. Unfortunately, however, as soon as the electron population in level 2 starts to increase, the pumping radiation induces stimulated emission from level 2 to level 1, since it is of the correct frequency and no build up occurs.
In a three level system, the pumping radiation of frequency (E3- E1)/h, raises electrons from level 1 to level 3, from which they fall by spontaneous emission to level 2. An inverted population can arise between level 2 and 1 if electrons remain long enough in level 2. The spontaneous emission of a photon due to an electronic fall from level 2 to level 1 may subsequently cause the stimulated emission of a photon which in turn releases more photons from other atoms. The laser action thus occurs between level 2 and 1 and the pumping radiation has different frequency from that o the stimulated radiation.
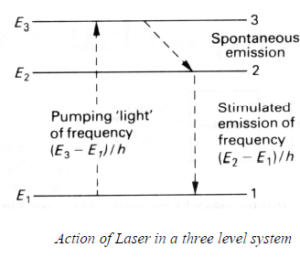
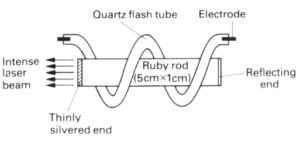
(b) Ruby Laser
Many materials can be used in laser. The ruby rod laser consists of a synthetic crystal of aluminium oxide containing a small amount of chromium as the laser material. It is a type of three-level leaser in which ‘level’3 consists of a band of very close energy levels. The pumping radiation, produced by intense flashes of yellow-green light from a flash tube, raises electrons from level 1 ( the ground level) into one of the levels of the band. From there they fall spontaneously to the metastable level 2 where they can remain for approximately 1 millisecond, as compared with 10-8 second in the energy band. Red laser light is emitted when they are stimulated to fall to level 1 from 2. One end of the ruby rod is silvered to act as a complete reflector whilst the other is thinly silvered and allow partial transmission. Stimulated light photons are reflected to and fro along the rod producing an intense beam, part of which emerges from the partially
silvered end as the useful output of the laser.
(c) Helium – neon laser.
This uses a mixture of helium and neon, and whereas the ruby laser emits short pulses of light, it works continuously and produces a less divergent beam. In one form the gas is in a long quartz tube with an optically flat mirror at each end. Pumping is done by a 28 M Hz r.f. generator instead of a flash tube. An electric discharge in the gas pumps the helium atoms to a higher energy level. They then excite the neon atoms to a higher level by collision and produce an inverted population of neon atom which emit radiation when they are stimulated to fall to a lower level.
(d). Uses.
Semiconductor lasers are used in optical fibre communication systems. Ruby lasers are used for range finding, welding, drilling and microcircuit fabrication. Helium-Neon lasers are used for the precision measurement of length, surveying, printing and holography.
