Phylum Anthophyta
Differences between Dicot and Monocot plants 



Beneficial characters obtained by Anthophyta due to evolution
Characters which promote anthophyta to become dominant over any of the other phyla included in kingdom Plantae
Elemental composition of living matter
All living matters are made up of many chemical compounds
Compounds form by the combination of many elements
Thus living organisms have chemical nature
Among the Ninety two naturally occurring elements about 20 – 25% of elements which constitute the living matter and are essential to continue healthy life and reproduce are called Essential elements (In human 25 & in plants 17)
C, H, N, O, P, S, K, Mg, Ca, Na, Cl, B, Mn, Zn, Cu, Mo, Si, V, I, Cr, Co (general)
Out of them Oxygen (O), Carbon (C), Hydrogen (H) and Nitrogen (N) make up 96% of living matter
Calcium (Ca), Phosphorus (P), Potassium (K) and Sulphur make up most of the remaining 4% of the mass of the organism
Based on the natural abundance of the essential elements in the living matter, can be divided into two
Macro Elements
Essential elements required by an organism in relatively high amount and found 0.01% or more in dry weight
Have low molecular weight (40)
Found in all elements C, H, N, O, P, S, K, Mg, Ca – 9
In addition to above some organisms (human) have Na, Cl, Fe
| Element | % in animal (human) | % in plant (Corn) | Function | |
| 01 | Oxygen | 65% | 44.4% | Major component of organic molecules |
| 02 | Carbon | 18.5% | 44% | Major component of organic molecules |
| 03 | Hydrogen | 9.5% | 6.24% | Major component of organic molecules |
| Component of amino acids | ||||
| Component of proteins | ||||
| Component of nucleotides | ||||
| Component of nucleic acids | ||||
| Component of coenzymes | ||||
| Component of enzymes | ||||
| Component of Chlorophyll | ||||
| 05 | Phosphorus | 1% | 0.2% | Component of ADP and ATP |
| Component of nucleic acids | ||||
| Component of phospholipids | ||||
| Component of several coenzymes | ||||
| 06 | Sulphur | 0.3% | 0.17% | Components of some amino acids and
proteins |
| Component of coenzyme – A | ||||
| 07 | Potassium | 0.4% | 0.92% | Protein synthesis |
| Operation of stomata | ||||
| 08 | Calcium | 1.5% | 0.23% | Component of cell walls |
| Maintenance of membrane structure and permeability | ||||
| Activates some enzymes. | ||||
| 09 | Magnesium | 0.1% | 0.18% | Component of chlorophyll molecule |
| Activates many enzymes |
Trace Elements
Essential elements required by an organism in minute quantities and found less than 0.01% in dry weight
B, Co, Cu, Cr, F, I, Fe, Mo, Mn, Se, Si, Sn, V, Zn
Some elements such as Iron (Fe) are required by all organisms while some are required only by certain species of animals (Iodine – Vertebrates)
| Element | Function |
| Sodium | · Osmosis |
| · Ionic balance | |
| · Nervous impulse transmission | |
| · Extracellular fluid | |
| Chlorine | · Extracellular fluid |
| · Enzyme activator | |
| · Osmosis | |
| · Ionic balance | |
| Iron | · Chlorophyll synthesis |
| · Component of cytochromes. | |
| · Component of nitrogenase | |
| · Component of blood pigments | |
| · Enzyme activator | |
| Manganese | · Activator of certain enzymes |
| Zinc | · Activator of many enzymes |
| · Activate formation of Chlorophyll | |
| Boron | · Carbohydrate transport |
| · Nucleic acid synthesis. | |
| Copper | · Activator or component of certain enzymes |
| Molybdenum | · Nitrogen fixation |
| · Nitrate reduction |
Elements in human body
Element Carbon
All living substances are made up of carbon
Because;
Physical and chemical properties of water important for life
Water is a vital inorganic molecule
Life could not exist on this planet without water. It is important due to following two reasons
Physical and chemical properties of water molecule provide the ability to render its vitality.
Water molecule is a small, bipolar and angular molecule (104.5°)
Polarity is an uneven charge distribution within a molecule. Hence in water molecule one pole is slightly positive and the other end is slightly negative. This condition is known as dipole. Due to this dipolarity water molecules have weak attraction for each other. This weak attractions are known as hydrogen bonds.
Main functions of water
Other functions of water
| Property | Role | Example |
| Liquid at room temperature | Medium of protoplasm | · Major component in protoplasm is H2O |
| Polarity | Powerful solvent | · Most of the materials of a cell are dissolved in protoplasm & cell sap
· Metabolic reactions take place in an aqueous medium in a cell |
| Chemical property | Reactant in some biochemical processes | · Photosynthesis
6CO2+6H2O → C6H12O6 + 6O2 · Hydrolysis Starch + H2O → Maltose |
| High adhesive and cohesive forces. | Maintenance of turgor in cells | · Cell enlargement
· mechanical support in herbaceous plants · Turgor movements · Movement of guard cells · Blooming of flowers |
| Transport and absorption of materials in organisms | · Translocation and ascent of sap
· Absorption of water and minerals from soil solution. |
|
| High surface tension | Provides habitats for some aquatic insects | · Water skaters |
| High specific heat capacity | Water resists to change its temperature when a considerable amount of heat is absorbed or lost | · Maintain the body temperature of poikilotherms within a narrow range |
| High latent heat of vaporization | Cooling the body surfaces | · Sweating
· Transpiration |
| High latent heat of fusion | A lot of heat should be dissipated for water bodies to freeze | · Water will not freeze easily within the cells and in water bodies. |
| Anomalous expansion of volume on freezing | Water bodies will not freeze solid. Ice forms on top and liquid water remains at the bottom | · Aquatic organisms are capable of surviving during winter. |
| Transparency | Allowing penetration of light | · Allows to grow aquatic plants and algae in a considerable depth in water bodies. |
Importance of water for life
Due to cohesion between water molecules, water and dissolved substances such as minerals and nutrients transport through vascular tissues, xylem and phloem against gravity
Due to adhesion between water molecules and cell walls also helps in conduction of water and dissolved substances
Due to the high specific heat, water will function as thermal buffer in living system and aquatic bodies during the temperature fluctuations on earth
Due to the high heat of vaporization, with the minimum loss of water an organism can loss much heat energy. Therefore body surface of an organism maintained as cool surface
Eg- evaporation of sweat from human skin keeps the body temperature at the constant level. Prevent from over heating
Transpiration in plants keeps the plant body surface and prevent from becoming too warm in the sunlight
Water can stretch maximum within a small area. This ability is given to water molecules, due to cohesion between the water molecules. Therefore in an aquatic system, upper surface water molecules are attracted by lower surface molecules and forms a water film. Small insects eg. Water skaters can walk on the surface of a pond.
Generally increase of the temperature of any substances reduces its density and decrease of the temperature of any substances increases its density. But for water when the temperature falls below 4°C it begins to freeze and forms a crystalline lattice called an ice cubes which increases the valence of few molecules of water and decreases its density. Therefore water has the maximum density at 4°C. Ice floats on the surface of water bodies. It is important in Polar Regions, organisms in the aquatic water bodies can survive during the winter.
This ability is given to water due to its polarity. Any polar molecules can dissolve in water because water molecules surround each of the solute molecules and form hydrogen bond with them. Solubility depends on the polarity. No need to be ionic to dissolve.
In some biochemical processes water is used as a reactant
In these reactions, water molecules act as hydrogen donors.
Organization of cells
Cells are the basic structural and functional unit of all organisms.
There are two kinds of cellular organization
Bacteria and Archaea are prokaryotic cells. All the other organisms have eukaryotic cells.
All cells share certain basic features. They are;
Difference between prokaryotic and eukaryotic cells
| Feature | Prokaryotic cells | Eukaryotic cells |
| Organisms | Bacteria
Archaea bacteria |
Protista, fungi, plants, animals |
| Cell size | Average diameter (1-5 micrometre) | 10-100 micrometre |
| Form | Mainly unicellular | Mainly multicellular (except Protista and fungi many of which are unicellular) |
| Evolutionary origin | 3.5 billion years ago | 1.8 billion years ago, evolved from prokaryotes |
| Cell division | Mostly binary fission
No spindle formation |
Mitosis, meiosis or both
Spindle form |
| Genetic material | DNA is circular and lies freely in the cytoplasm
This region is called nucleoid. DNA is naked, not associated with proteins or RNA to form chromosomes |
DNA is linear and contained in a nucleus.
DNA is associated with proteins and RNA to form chromosomes |
| Type of ribosomes | 70s ribosomes (smaller) | Both 70s and 80s ribosomes (larger) present, may be attached to endoplasmic reticulum |
| Organelles | Few organelles, none are surrounded by membrane.
Internal membranes scares; if present usually associated with respiration, photosynthesis and N2 fixation |
Many organelles, membrane bounded organelles found. Great diversity of organelles. E.g.-
· Nucleus, mitochondria, chloroplasts bound by two membranes. · Lysosomes, vacuole, microbodies are bounded by single membrane |
| Cell walls | Bacteria – Peptidoglycan
Archea – Polysaccharide and protein |
Cell walls of plants and fungi are rigid and contain polysaccharides; cellulose is the main strengthening compound of plant cell walls
Chitin of fungal walls Cell wall is absent in animal cell |
| Flagella | Simple, lacking microtubules; extracellular.
Not enclosed by cell surface membrane 20nm diameter |
Complex with 9+2 arrangement of microtubules.
Inter cellular, surrounded by cell surface membrane 200nm diameter |
| Respiration | Mesosomes in aerobic bacteria, except cytoplasmic membranes in cyanobacteria | Mitochondria for aerobic respiration |
| Photosynthesis | No chloroplasts.
Take place on membranes which show no stacking |
Chloroplasts containing membranes which are usually stacked into lamella or grana |
| Nitrogen fixation | Some have the ability | Non have the ability |
Animal tissue
Animal body is made up of four main types of tissues;
Tissues
Plant tissues
Classified into two based on the nature of dividing ability of the cells
1.Meristamatic tissue
e.g. – apical meristem, cambium
Features-
2. Permanent tissue
2. Complex permanent tissue– have cells which are of different types
Cell specialisation
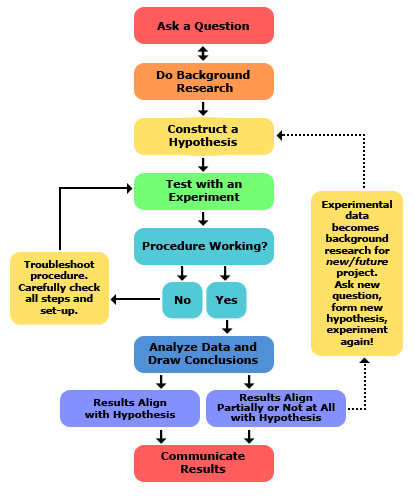
An organized pattern of investigation or standard sequence of steps normally followed by scientists or biologists all over the world in investigating particular event or problem
Steps include :
A cheque can be defined as a written order made by a current account holder (Drawer) to a bank. (Drawee)
The main features of a cheque
• Date
• Name of bank and branch
• The name of payee
• The term “Or bearer”
• The amount of money in words and figures.
• The term “Pay”
• Signature of account holder
• Magnetic recognition strip (Cheque no: Bank no, Branch no, and Account no.)
The different parties involved in a cheque
• Current account holder (drawer)
• Payee
• Drawee/ Bank
Cheques can be catergorized as follows
• Bearer cheque
• Order cheque
Factors taken into consideration when writing a cheque
• Use indelible ink when writing a cheque
• Note the relevant details on counter foil.
• Draw one line across any mistake you make and place full signature of the drawer
• Sign correctly according to the specimen signature
• Write the correct date and use safety method.
• No blank cheques should be signed.
A Crossed cheque
It is a cheque where two parallel alines are drawn across its face or the name of a commercial bank is stated called a crossed cheque
• A cheque crossed for security
• There are 2 types of crossed cheques . They are general crossing and special crossing.
• A general crossed cheque is not negotiable or Two parallel lines drawn on the face of a cheque
A special crossed cheque refers to the name of a commercial bank with or without the parallel lines.
An endorsement is where you write your name on the back of the cheque as it appears on the face of the cheque. The endorsement will be made in the following instances
• When it is deposited in the bank
• When a cheque is transferred to another person
• Non – crossed cheques – encased over the counter
• When a bank refuses payment for a cheque it is called dishonoring
The reasons for dishonoring a cheque
• Stop payment
• The death of the account holder and the bank has been informed.
• Insolvency/ bankruptcy
• Court order
• Wrongly written
• Insufficient funds