sub-atomic paticles
Cathode rays

Discharge tube
Discharge tube is also called “CROOK TUBE”. It is made of a glass tube which consists of two metallic plates. One plate is connected to positive terminal of high voltage power supply and the other to negative terminal. The plate connected to the positive terminal is called “ANODE” the other connected to negative terminal is called “CATHODE”. The tube is filled with any gas.
Experiment
In discharge tube experiment, at low pressure and at very high voltage, an electric current is passed. Due to passage of electric current, a stream of rays is passed in the tube originating from cathode. These rays are called “CATHODE RAYS”.
Properties of cathode rays
Isotopes
The atoms which have the same atomic number and different mass numbers are defined as isotopes of that element.
Nuclides
Atomic species of which the proton number and nucleon number is specified.
Type of nuclides 1. Stable nuclides existing in nature 2. Unstable nuclides existing in nature 3. Artificial radioactive nuclides
Radioactivity
Emission of radiation by unstable nuclei or particles spontaneously for the sake of stability of the nucleus is called radioactivity. These can penetrate and ionize gases.
Properties of alpha, beta and gamma rays
Thomson’s atomic model
Earliest theoretical description of the inner structure of atoms, proposed about 1900 by Lord Kelvin and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom. Though several alternative models were advanced in the 1900s by Lord Kelvin and others, Thomson held that atoms are uniform spheres of positively charged matter in which electrons are embedded. Popularly known as the plum-pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the Rutherford atomic model.
Rutherford’s model
Rutherford overturned Thomson’s model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, heavy nucleus. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure.
Gold foil experiment
At Rutherford’s behest, Geiger and Marsden performed a series of experiments where they pointed a beam of alpha particles at a thin foil of metal and measured the scattering pattern by using a fluorescent screen. They spotted alpha particles bouncing off the metal foil in all directions, some right back at the source. This should have been impossible according to Thomson’s model; the alpha particles should have all gone straight through. Obviously, those particles had encountered an electrostatic force far greater than Thomson’s model suggested they would, which in turn implied that the atom’s positive charge was concentrated in a much tinier volume than Thomson imagined.
When Geiger and Marsden shot alpha particles at their metal foils, they noticed only a tiny fraction of the alpha particles were deflected by more than 90°. Most just flew straight through the foil. This suggested that those tiny spheres of intense positive charge were separated by vast gulfs of empty space.Most particles passed through the empty space and experienced negligible deviation, while a handful struck the nuclei of the atoms and bounced right back.
Rutherford thus rejected Thomson’s model of the atom, and instead proposed a model where the atom consisted of mostly empty space, with all its positive charge concentrated in its center in a very tiny volume, surrounded by a cloud of electrons.
Bohr model and postulates
Bohr model is based on three postulates. 1. Only orbits of certain radii, corresponding to certain definite energies, are permitted for the electron in a hydrogen atom. 2. An electron in a permitted orbit has a specific energy and is in an “allowed” energy state. An electron in an allowed energy state will not radiate energy and therefore will not spiral into the nucleus. 3. Energy is emitted or absorbed by electron only as the electron transfers from one allowed energy state to another. This energy is emitted or absorbed as a photon, E = h υ .
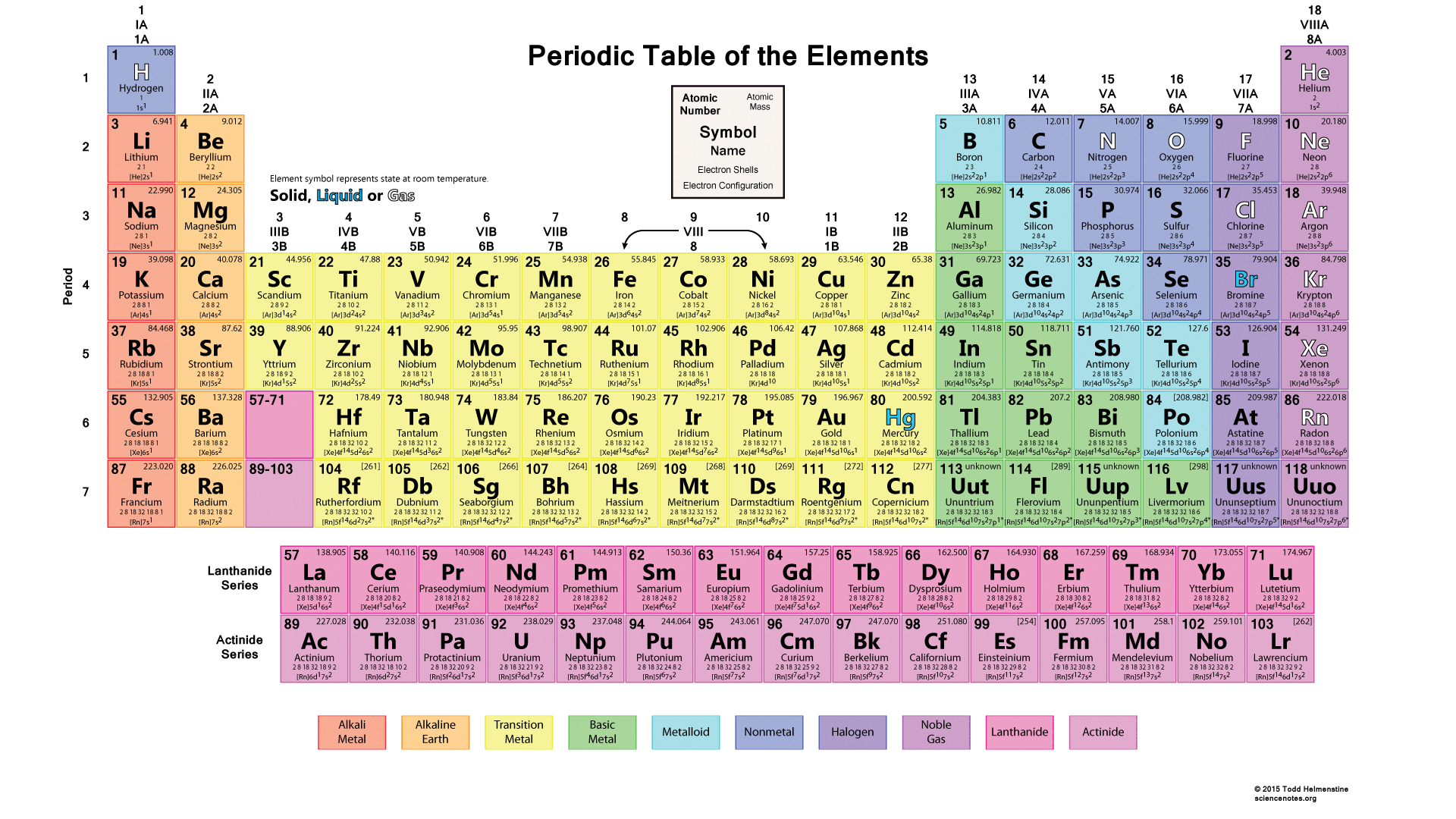
Formation of cations and anions
• Formation of anions and cations depends on the number of electrons in the valency shell and ionization energy. • The elements of the group I(1), II(2) and III(13), form cations while the elements of the groups V(15), VI(16) and VII(17) form anions. • The elements in the group IV(14) generally do not form free M4+ ions. The reason is the high aggregate of first, second, third and fourth ionization energies.
Oxidation states
• In the elemental state oxidation number of any element is considered as zero. • Oxidation state is a measure of the electron control that an atom has in a compound compared to its elemental state. • The highest oxidation number that an element can have in a compound is equal to its number of valency electrons. • In combined state certain elements have variable oxidation states.
Oxidizing ability
• In general oxidizing ability of elements decreases across the period up to group 17. • In general oxidizing ability of elements increases down the group.
Electronegativity
• The ability of an element to attract electrons in a bond of a molecule towards itself varies from one element to the other. When expressed quantitatively, this ability is known as the electronegativity of an element.
• Electronegativity is expressed according to various scales. The following table gives the electronegativity values for different elements according to the Pauling scale in their most common oxidation state.
Although a different value for the electronegativity of each element is stated according to the Pauling scale, the electronegativity of an atom of an element changes according to its environment (Hybridization, Charge, Oxidation number). Example : In the species NH2– , NH3 , NH4+ and the electronegativity of N varies in the order NH2– < NH 3< NH4+
Electron affinity
• It is the change in energy that takes place when a mole of uni- negative ions are formed in the gaseous state when electrons are given to a mole of atoms in the gaseous state. • The first electron affinity of many elements takes a negative value. It is because the added electron gets attracted by the nucleus. The second electron affinity always takes a positive value. It is because an electron is added to an already negative ion.
•Across a period from left to right the nuclear charge increases and atomic radius decreases. Hence, the ionization energy increases. Therefore, the tendency to form cations decreases and also the ability to act as reducing agents decreases across a period. •Similarly the ability to form anions increases and also the ability to act as oxidizing agent increases from left to right across a period.
Atomic radius
• In general, the atomic radius is referred as the distance between the nucleus and the outermost energy level occupied by electrons. • However, the position of an electron is uncertain and consequently it is difficult to express the radius of an atom. • Therefore, atomic radius is expressed in different ways.
• Attraction towards outermost electrons from the nucleus is hindered to a certain extent by the electrons existing in inner energy levels. This effect is referred as ‘shielding effect’. • Protons in the nucleus attract the electron cloud. The resultant effect of this attraction and shielding effect is referred as ‘effective nuclear charge’. • Shielding effect affects the atomic radius and ionization energy.
Covalent radius
When two atoms of the same element are covalently bonded, half the internuclear distance of these two atoms is called its covalent radius. Covalent radius = d/2 • The covalent atomic radius increases down a group and decreases from left to right along a period up to group 18.
Van der Waals radius
When two molecules or atoms are placed as close together as possible, half the distance between the two nuclei which are close to each other is called the Van der waals radius. Van der Waals radius = d/2
Metallic radius
Half the distance between two adjacent cation nuclei in the metallic lattice is the metallic radius. metallic radius = d/2 • Ionization energy is determined by nuclear charge, radius and shielding effect.
Ionic radius
• A value assigned to the radius of an ion in a crystalline solid, based on the assumption that the ions are spherical with a definite size. X-ray diffraction can be used to measure the inter-nuclear distance in crystalline solids. Ionic radius can be calculated according to the inter-nuclear distance. • In general, negative ions have larger ionic radii than their neutral atoms and positive ions have smaller ionic radii than their neutral atoms.
Hund’s rule
Orbitals of the same energy (degenerate) are occupied by electrons singly so that their spins are parallel to make the maximum number of unpaired electrons and then doubly with their spins anti parallel.
Pauli’s exclusion principle
This principle states that no orbital can accommodate more than two electrons (In other words the set of quantum numbers for a certain electron of an atom is exclusive for it or no two electrons in an atom can have the same set of quantum numbers.)
Aufbau principle
It states that the filling up of electrons in the orbitals takes place according to the increasing order of energy of the orbitals in accordance with the Pauli exclusion principle.
Ascending order of energy in the sub energy levels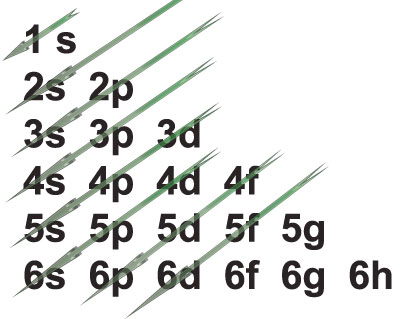
Graph showing the variation of first ionization energies of elements of atomic number
Anomalous behaviour seen from group II to III and from group V to VI is due to extra stability of half-filled (p³ ) and completely-filled shells (s² , p⁶ ). Electronic configurations d⁵ and d¹⁰ also exhibit extra stability.
• Energy is transmitted as electromagnetic radiation through the space. • They consist of both electric and magnetic fields which are perpendicular to each other. • Velocity of all the electromagnetic radiation in vacuum is; 3.00 x 10⁸ m s-1 which is the velocity of light. • When is the wave length and is the frequency, the velocity of an electromagnetic wave- C = υλ. • The energy of an electromagnetic wave E = hυ (E is the energy of a photon.) where h is a constant. It is named as Planck’s constant. Planck constant = 6.624 x 10-34 J s
Arrangement of the electromagnetic radiations in the ascending order of the frequency produces the electromagnetic spectrum.
Uses of the radiations belonging to different ranges of the electromagnetic spectrum
• Radio waves : Used for communication through television and radio media.
• Radar waves : Used in naval and aeronautical security systems.
• Micro waves : Microwave ovens function due to these waves. Used in cellular phones.
• Infrared waves : Used in physiotherapy treatments. Used in sending signals by remote control devices and also in analytical work using spectroscopic methods.
• Visible waves : Vision and photography are due to waves in this range. Used in colorimetric analysis.
• Ultraviolet waves : Used for sterilization and to read confidential symbols in currency notes etc. Also used in spectroscopic analysis.
• X- rays : Used in X ray photography and in studies of the structure of crystals etc.
• γ rays : Used in the treatment of cancer.
1.Variation of successive ionisation energies
The above graph illustrates all the successive ionization energies of chlorine.The number of successive ionisation energies is equal to the number of electrons in the atom. When attention is paid to sudden increase in successive ionisation energies it is proved that electrons exist as groups in various energy levels.
2.Absorption spectrum
3.Emission spectrum
Limitations of Bohr model
While the Bohr model explains the line spectrum of the hydrogen atom, it cannot explain the spectra of other atoms, except in a rather crude way. Bohr also avoided the problem why the negatively charged electrons would not just fall into the positively charged nucleus by simply assuming it would not happen. Therefore, there is a problem with describing an electron merely as a small particle circling about the nucleus.
s, p, d and f sub-energy levels
Shapes of orbitals
Wave – particle nature of electrons
• Wave properties : While passing through an ionic crystal a beam of electrons gets diffracted in the same way as a beam of X-rays does. A beam of electrons also show interference patterns. • Particle properties : A beam of electrons has ability to do work (due to the momentum) and also it has a charge.
Quantization of energy
• Atoms absorb or emit energy in the form of definite small quantities. • The smallest quantity of energy is referred as ‘quantum’ or ‘photon’. • According to Planck, matter absorbs or emits energy in the form of photons or whole number multiples of it. eg. hυ , 2 hυ , 3 hυ , …………. • Hence, it is considered as energy is quantized.
• Principal quantum number (n)
The main energy level to which the electron belongs is represented by this quantum number. n = 1, 2, 3, …..
• Azimuthal quantum number (l)
The sub – energy level (s,p,d,f….) to which the electron belongs is represented by this quantum number. l = 0, 1, 2, ……, (n-1)
• Magnetic quantum number (ml )
The orbital (Eg. p x , p y , p z ) in which the electron exists in a certain sub- energy level is represented by this quantum number ml = -l, (-l+1),….,0,…..,(l-1), l
• Spin quantum number (ms )
The direction of spin of an electron present in a certain orbital is represented by this quantum number. ms = ± ½
Assignment of the four quantum numbers n, l, ml and ms for electrons in the first four energy levels of an atom