Aluminum (also called Aluminium) is the third most abundant element in the earth’s crust. It is commonly used in the household as aluminum foil, in crafts such as dyeing and pottery, and also in construction to make alloys. In its purest form the metal is bluish-white and very ductile. It is an excellent conductor of heat and electricity and finds use in some wiring. When pure it is too soft for construction purposes but addition of small amounts of silicon and iron hardens it significantly.
The electron configuration for Aluminum is 1s22s22p63s23p1.
Aluminum has three oxidation states. The most common one is +3. The other two are +1 and +2.
Reaction of aluminium with air
Aluminium is a silvery white metal. The surface of aluminium metal is covered with a thin layer of oxide that helps protect the metal from attack by air. So, normally, aulumium metal does not react with air. If the oxide layer is damaged, the aluminium metal is exposed to attack. Aluminium will burn in oxygen with a brilliant white flame to form the trioxide alumnium(III) oxide, Al2O3.
4Al(s) + 3O2(l) → 2Al2O3(s)
Reaction of aluminium with water
Aluminium is a silvery white metal. The surface of aluminium metal is covered with a thin layer of oxide that helps protect the metal from attack by air. So, normally, aulumium metal does not react with air. If the oxide layer is damaged, the aluminium metal is exposed to attack, even by water.
Reaction of aluminium with the halogens
Aluminium metal reacts vigorously with all the halogens to form aluminium halides. So, it reacts with chlorine, Cl2, bromine, I2, and iodine, I2, to form respectively aluminium(III) chloride, AlCl3, aluminium(III) bromide, AlBr3, and aluminium(III) iodide, AlI3.
2Al(s) + 3Cl2(l) → 2AlCl3(s) → Al2Cl6(s)
AlCl3 is an electron defficient compound and also highly covalent when anhydrous. AlCl3 exists as a dimer thus attaining an octet of electrons.
2Al(s) + 3Br2(l) → Al2Br6(s)
2Al(s) + 3I2(l) → Al2I6(s)
Reaction of aluminium with acids
Aluminium metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated Al(III) ion together with hydrogen gas, H2. The corresponding reactions with dilute hydrochloric acid also give the aquated Al(III) ion. Concentrated nitric acid passivates aluminium metal.
2Al(s) + 3H2SO4(aq) → 2Al3+(aq) + 2SO42-(aq) + 3H2(g)
2Al(s) + 6HCl(aq) → 2Al3+(aq) + 6Cl–(aq) + 3H2(g)
Reaction of aluminium with bases
Aluminium dissolves in sodium hydroxide with the evolution of hydrogen gas, H2, and the formation of aluminates of the type [Al(OH)4]–.
2Al(s) + 2NaOH(aq) + 6H2O → 2Na+(aq) + 2[Al(OH)4]– + 3H2(g)
Carbon is a Group 14 element and is distributed very widely in nature. It is found in abundance in the sun, stars, comets, and atmospheres of most planets. Carbon is present as carbon dioxide in the atmosphere and dissolved in all natural waters. It is a component of rocks as carbonates of calcium (limestone), magnesium, and iron.
Coal, petroleum, and natural gas are chiefly hydrocarbons. Carbon is unique among the elements in the vast number of variety of compounds it can form.
Carbon is found free in nature in three allotropic forms: amorphous, graphite, and diamond. Graphite is one of the softest known materials while diamond is one of the hardest
More recently, another form of carbon, buckminsterfullerene, C60, was discovered. This form of carbon is the subject of great interest in research laboratories today.
Pure carbon is available in a number of different forms (allotropes). The most common form of pure carbon is α-graphite. This is also the thermodynamically most stable form. Diamond is a second form of carbon but is much less common. Other forms of carbon include the fullerenes. Whereas diamond and graphite are infinite lattices, fullerenes such as buckminsterfullerene, C60, is a discrete molecular species. Amorphous forms of carbon such as soot and lampblack are materials consisting of very small particles of graphite.

Atom arrangements in the most common allotrops of carbon: α-graphite.
As diamond has a slightly more compact structure its density is greater than that of graphite. The appearance of diamond is well known and it is also one of the hardest materials known. Like graphite, it is relatively unreactive but does burn in air at 600-800°C. Each carbon atom is bound to four neighbours at a distance of 154.45 pm in a tetrahedral fashion and so each diamond crystal is a single giant lattice structure. In principle (and in practice!) graphite may be converted into diamond by the application of heat and pressure.

Crystal strucutres of diamond.
Recently another allotrope of carbon was characterized. Whereas diamond and graphite are infinite lattices, buckminsterfullerene, C60, is a discrete molecular species. The buckminsterfullerene molecule is a net of 12 pentagons and 20 hexagons folded into a sphere. The effect is very similar to the patchwork of 12 pentagonal and 20 hexagonal pieces of leather that sewn together make up an association football (soccer ball). The name buckminsterfullerene (or buckyball was coined because of the relationship between the structure of C60 and R. Buckminster Fuller’s geodesic dome designs. Buckminsterfullerene is now commercially available and has also been identified in interstellar space and soot.
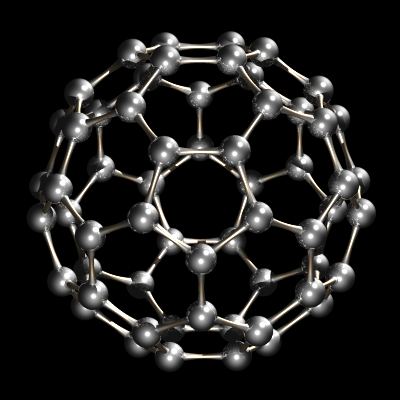
C60, Buckminsterfullerene.
Nanotubes are related to fullerenes. They are tubes giving the appearance of rolled graphite, although they are made from graphite. They are open ended while fullerenes are closed structures.
Oxides of carbon
CO is a colourless, neutral, and poisonous gas. CO is used as an industrial fuel. CO is a Lewis acid.
CO2 is a colourless acidic gas and a non polar molecule. Solid CO2 (dry ice) contains dispersion interactions. Dry ice is used as a freezing agent in food industry, in causing artificial rain, etc.
Carbonic acid
Carbonic acid is weak diprotic acid. There are two salts derived from H2CO3 .
CO2 + H20 → H2CO3
H2CO3 → H+ + HCO3–
HCO3– → H+ + CO32-
Reaction of carbon with air
Carbon, as graphite, burns to form gaseous carbon (IV) oxide (carbon dioxide), CO2. Diamond is a form of carbon and also burns in air when heated to 600-800°C – an expensive way to make carbon dioxide!
C(s) + O2(g) → CO2(g)
When the air or oxygen supply is restricted, incomplete combustion to carbon monoxide, CO, occurs.
2C(s) + O2(g) → 2CO(g)
This reaction is important. In industry, air is blown through hot coke. The resulting gas is called producer gas and is a mixture of carbon monoxide (25%), carbon dioxide (4%), nitrogen (70%), and traces of hydrogen (H2), methane (CH4), and oxygen (O2).
Reaction of carbon with water
Carbon, either as graphite or diamond does not react with water under normal conditions. Under more forsing conditions, the reaction becomes important. In industry, water is blown through hot coke. The resulting gas is called water gas and is a mixture of hydrogen (H2, 50%), carbon monoxide (CO, 40%), carbon dioxide (CO2, 5%), nitrogen and methane (N2 + CH4, 5%). It is an important feedstock gas for the chemical industry.
C + H2O → CO + H2
This reaction is endothermic (ΔH° = +131.3 kJ mol-1; ΔS° = +133.7 J K-1 mol-1) which means that the coke cools down during the reaction. To counteract this, the steam flow is replaced by air to reheat the coke allowing further reaction.
Reaction of carbon with the halogens
Graphite reacts with fluorine, F2, at high temperatures to make a mixture of carbon tetrafluoride, CF4, together with some C2F6 and C5F12.
C(s) + excess F2(g) → CF4(g) + C2F6 + C5F12
At room temperature, the reaction with fluorine is complex.
The other halogens appear to not react with graphite.
Carbon has a very high melting and boiling point and rapidly combines with oxygen at elevated temperatures. In small amounts it is an excellent hardener for iron, yielding the various steel alloys upon which so much of modern construction depends. An important (but rare) radioactive isotope of carbon, C-14, is used to date ancient objects of organic origin.
N2 is a colourless gas. It contains a triple bond, , with a short bond length of 1.09 °A. It has high dissociation bond energy 946 kJ mol-1 and it is an inert gas.
• Liquid N2 (boiling point -196 °C) is used as a coolant. N2 gas is used in the manufacture of ammonia.
• Nitrogen shows variable oxidation states.
-3 -2 -1 0 +1 + 2 +3 +4 +5
NH3 N2H4 NH2OH N2 N2O NO N2O3 N2O4 N2O5
Oxo acids of nitrogen
Nitrous acid (HNO2 ) is unstable except in dilute solutions. HNO2 is formed when acid reacts with metal nitrites.
NaNO2(s) + HCl(aq) → NaCl(aq) + HNO2(aq)
Nitric acid (ΗΝΟ3 ) is a colourless liquid which boils at 86 °C. ΗΝΟ3 a stable, strong acid as well as a strong oxidizing agent. Concentrated ΗΝO3 acid is usually yellow. In the light, it decomposes to form nitrogen dioxide and oxygen.
4HNO3(l) → 4NO2(g) + O2(g) + 2H2O(l)
Compounds
The two most common compounds of nitrogen are Potassium Nitrate (KNO3) and Sodium Nitrate (NaNO3). These two compounds are formed by decomposing organic matter that has potassium or sodium present and are often found in fertilizers and byproducts of industrial waste.
Nitrides are compounds of nitrogen with a less electronegative atom; in other words it’s a compound with atoms that have a less full valence shell. These compounds form with lithium and Group 2 metals. Nitrides usually has an oxidation state of -3
3Mg+ N2→Mg3N2
Nitrogen goes through fixation by reaction with hydrogen gas over a catalyst. This process is used to produce ammonia. As mentioned earlier, this process allows us to use nitrogen as a fertilizer because it breaks down the strong triple bond held by N2. The famous Haber-Bosch process for synthesis of ammonia looks like this:
N2+3H2→2NH3
Ammonia is a base and is also used in typical acid-base reactions.
ΝH3 acts as an oxidizing agent and as well as an acid
2Na(s) + 2NH3(l) → 2NaNH2(s) + H2(g)
ΝH3 acts as a weak reducing agent.
3NH3(l) + 2Cl2(g) → 2 N2(g) + 6HCl(g)
3CuO(s) + 2NH3(l) → N2(g) + 3Cu(s) + 3H2O(l)
ΝH3 acts as a weak reducing agent.
2NH3(aq)+H2SO4→(NH4)2SO4(aq)
Ammonium salts
Ammonium salts decompose quite readily on heating.
(NH4)2CO3(s) →Δ 2NH3(g) + CO2(g) + H2O(l)
NH4Cl(s) →Δ NH3(g) + HCl(g)
NH4NO2(s) →Δ N2(g) + 2H2O(l)
NH4NO3(s) →Δ N2O(g) + 2H2O(l)
(NH4)2Cr2O7(s) →Δ N2(g) + Cr2O3(s) + 4H2O(s)
Nitrides use a variety of different oxidation numbers from +1 to +5 to for oxide compounds. Almost all the oxides that form are gasses, and exist at 25 degrees Celsius. Oxides of nitrogen are acidic and easily attach protons.
N2O5+H2O→2HNO3(aq)
The oxides play a large role in living organisms. They can be useful, yet dangerous.
Hydrides of nitrogen include ammonia (NH3) and hyrdrazine (N2H4).
Allotropes of Oxygen
There are two allotropes of oxygen; dioxygen (O2) and trioxygen (O3) which is called ozone. The reaction of converting dioxygen into ozone is very endothermic causing it to occur rarely and only in the low atmosphere. The reaction is shown below:
3O2(g)→2O3(g)
Ozone is unstable and quickly decomposes back to oxygen but is a great oxidizing agent
Group 17 elements (halogens) fluorine, chlorine, bromine, and iodine react with oxygen to form oxides. Fluorine forms two oxides with oxygen which are F2O and F2O2. Both fluorine oxides are called oxygen fluorides because fluorine is the more electronegative element. One of the fluorine reactions is shown below.
O2(g)+F2(g)→F2O2(g)
Rhombic Sulphur (Octahedral or Alpha Sulphur)
Rhombic sulphur is prepared by dissolving roll sulphur in carbon disulphide, and then evaporating the solution slowly, at room temperature.Rhombic or octahedral sulphur consists of rings of 8 atoms of sulphur. It is the most stable of all the allotropes of sulphur. It is soluble in carbon disulphide, benzene, chloroform, etc., but is insoluble in water. It is non-conductor of heat and electricity. It is transparent and pale yellow in color.
Monoclinic Sulphur (Prismatic or Beta Sulphur)
This allotrope of sulphur is prepared by melting roll sulphur in a dish. The molten sulphur is allowed to cool slowly. The top layer solidifies first and forms a crust. Two holes are made in the crust with the help of a heated nail. The molten sulphur is poured out through one of the holes. Then with the help of a knife the crust is carefully peeled off
Pale-yellow, transparent needle shaped crystals are seen projecting out form the inner surface of the dish. These are the crystals of monoclinic sulphur. Monoclinic sulphur also consists of 8 atom rings. It is stable only above 96oC. When it cools down below 96oC, it changes to rhombic sulphur i.e., 96oC is the transition temperature of this sulphur.
Plastic Sulphur
On heating sulphur, till almost the boiling point and suddenly cooling it by pouring into cold water a viscous mass is formed. This sudden cooling does not allow sufficient time to the molecules to rearrange themselves to form monoclinic or rhombic forms of sulphur. Hence the molecules form an interwined mass, consisting of both rhombic and monoclinic varieties of sulphur. This is called plastic sulphur
This type of sulphur is a dark brown or even black, sticky substance. It is elastic. It has no sharp melting point. It does not dissolve in carbon disulphide. On standing, it slowly changes to the rhombic forms, as it gains the eight atom ring structure.
Colloidal Sulphur
This type of sulphur is prepared by passing hydrogen sulphide through a cooled saturated solution of sulphur dioxide in water, or by adding a solution of sulphur and alcohol in water. Colloidal sulphur is soluble in carbon disulphide. It is used in medicine.
Magnesium burns in sulphur di oxide giving magnesium oxide and sulphur.


Potassium reacts with sulphur dioxide forming potassium sulphite and potassium thiosulphate.


Sulphur dioxide reacts with alkali solution to give salt and water. [sulphites and bisulphites] When sulphur di oxide is bubbled through sodium hydroxide solution no precipitate is formed since sodium sulphite is a soluble salt.When sulphur dioxide is passed through Ca (OH)2 solution, a white precipitate of insoluble calcium sulphite is formed. If excess sulphur dioxide is passed, the precipitate disappears forming soluble calcium bi sulphite.











Oxidises magnisium to magnisium ion(Mg+2)
 Oxidises hydrogen silphide to sulphur
Oxidises hydrogen silphide to sulphur

In the presence of moisture SO2 liberated nascent hydrogen and reduction takes place by addition of hydrogen.
Example:


Potassium dichromate, potassium permanganate and nitric acid are reduced by the action of SO2 by removal of oxygen.Example:


 Sulphur dioxide gas exhibits bleaching properties in presence of moisture. It dissolves in water liberating nascent hydrogen. Coloring matter is bleached by reaction with nascent hydrogen. Nascent hydrogen removes oxygen atoms from the coloring matter (reduces coloring matter) and it loses its color. This bleaching is temporary because the bleached product on exposure to atmospheric oxygen adds on oxygen atoms from air and regains its original color.
Sulphur dioxide gas exhibits bleaching properties in presence of moisture. It dissolves in water liberating nascent hydrogen. Coloring matter is bleached by reaction with nascent hydrogen. Nascent hydrogen removes oxygen atoms from the coloring matter (reduces coloring matter) and it loses its color. This bleaching is temporary because the bleached product on exposure to atmospheric oxygen adds on oxygen atoms from air and regains its original color.
Uses of sulphur dioxide
The various uses of sulphur dioxide are:
1) In the manufacturing of sulphuric acid, sulphites, and hydrogen sulphite.
2) In the sugar industry for refining and decolorizing sugar.3) For refining kerosene, and other petroleum products.
4) As a disinfectant.5) As a fumigant.
6) For bleaching delicate articles.7) As antichlor, to remove the excess chlorine from substances that have been bleached by chlorine.
8) As a solvent for glue.9) As a refrigerant in household refrigerators.
10) As a preservative for wines, meat, dry fruits etc.
Sulphuric acid, H2SO4 is a colorless, odorless, extremely corrosive, oily liquid. It was initially called oil of vitriol.
Uses of sulphuric acid
This all means that oxidising ability falls as you go down the Group.
Position of noble gas in periodic table
Noble gases are also known as inert gases and do not take part in chemical reactions. They have their outermost shell complete and thus remain stable. They do not generally combine with other substances, nor are they affected by oxidising agents or by reducing agents. They are placed in the 18 or VIIIA group. Since, the outermost shell is complete, the valency is zero, hence VIIIA group is also referred to as zero group.
occurrence of noble gas
Noble gases always occur in free state because of their inert nature. All the noble gases, except radon are present in air in small amounts. The relative abundance of the noble gases in air is = 1%. Helium is present in natural gas up to the extent of 10 per cent. It is also present in small quantities in the minerals of radioactive elements. Water from certain springs also gives off gases, which are rich in helium and argon.
Uses of noble gas
Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes (arc welding of metals or alloys) and for filling electric bulbs. It is also used in the laboratory for handling substances that are air-sensitive.
Helium is a non-flammable and light gas. Hence, it is used in filling balloons for meteorological observations. It is also used in gas-cooled nuclear reactors. Liquid helium (boiling point 4.2K) finds use as cryogenic agent for carrying out various experiments at low temperatures. It is used to produce and sustain powerful super conducting magnets, which form essential part of modern NMR spectrometers and Magnetic Resonance Imaging (MRI) systems for clinical diagnosis.
Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. There are no significant uses of xenon and krypton. They are used in light bulbs designed for special purposes.
First ionization energy graph of elements from H to Cs
Variation of metalic radius, electronnegativity and ionisation energy from Sc to Zn
Physical properties of elements
| Z and symbol | 21 Sc | 22 Ti | 23 V | 24 Cr | 25 Mn | 26 Fe | 27 Co | 28 Ni | 29 Cu | 30 Zn |
| property\name | scandium | titanium | vanadium | chromium | manganese | iron | cobalt | nickel | copper | zinc |
| melting point/oC | 1541 | 1668 | 1910 | 1857 | 1246 | 1538 | 1495 | 1455 | 1083 | 420 |
| density/gcm–3 | 2.99 | 4.54 | 6.11 | 7.19 | 7.33 | 7.87 | 8.90 | 8.90 | 8.92 | 7.13 |
| atomic radius/pm | 161 | 145 | 132 | 125 | 124 | 124 | 125 | 125 | 128 | 133 |
| M2+ ionic radius/pm | na | 90 | 88 | 84 | 80 | 76 | 74 | 72 | 69 | 74 |
| M3+ ionic radius/pm | 81 | 76 | 74 | 69 | 66 | 64 | 63 | 62 | na | na |
| common oxidation states | +3 only | +2,3,4 | +2,3,4,5 | +2,3,6 | +2,3,4,6,7 | +2,3,6 | +2,3 | +2,+3 | +1,2 | +2 only |
| outer electron configuration [Ar]… | 3d14s2 | 3d24s2 | 3d34s2 | 3d54s1 | 3d54s2 | 3d64s2 | 3d74s2 | 3d84s2 | 3d104s1 | 3d104s2 |
Oxidation numbers of elements from Sc to Zn (Common oxidation numbers are shown in bold letters)
Transition ions formed by d block metals have partially filled d orbitals. These ions absorb selected range of wave length in white light and get excited and shows the complementary colours. However, d0 and d10 confingurations are colourless.
Sc3+ – Colourless
Co2+ – Pink
Ti4+ – Colourless
Ni2+ – Green
Ti3+ – Purple
Cu2+ – Blue
V3+ – Green
Cu+ – Colourless
V2+ – Violet
Zn2+ – Colourless
Cr3+ – Purple
Mn3+ – Violet
Mn2+ – Pale pink
Fe3+ – Brown yellow
Fe2+ – Pale green
Colours of some oxoanions: Usually, d element oxoanions are coloured.
MnO4– – Purple/Violet
MnO42- – Green
CrO42- – Yellow
Cr2O72- – Orange
Complex ions formed by the elements Cr, Mn, Fe, Co, Ni and Cu with the ligands H2O, NH3 and Cl–
• OH-acts as a ligand in few d block cations.
eg. [Fe(H2O)5OH]2+ formed by the hydrolysis of hydrated Fe3+ ions. However, most
metal ions with NaOH or NH3 give insoluble hydroxides and not hydroxo complexes.
eg. Cr(OH)3 – Green, Fe(OH)3 – Reddish brown, Fe(OH)2 – Dirty green, Cu(OH)2 – Blue, Mn(OH)2 – White
• The colour of the complex varies depending on the central metal atom.
eg. [Cr(H2O)6]3+ – Blue – violet
[Fe(H2O)6]3+ – Yellow
• The colour of the complex varies depending on the oxidation state of the central metal atom.
eg. [Fe(H2O)6]2+ , Pale green
[Fe(H2O)6]3+ – Yellow
[Mn(H2O)6]2+ – Pale pink
[Mn(H2O)6]3+ – Violet
• The colour changes also when the ligands change.
eg. [Co(H2O)6]2+ – Pink
[Co(NH3)6]2+ – Yellow brown
• Here, the information required to develop the IUPAC name of a complex and to write the structural formula when the IUPAC name is given will be discussed. Only complexes formed by elements of the d block are considered.
The complexes are considered simply under two categories.
(i) Cations are simple while the anions are complex.
(ii) Cations are complex while the anions are simple.
• Whatever the complex considered, a common set of rules has to be followed stepwise in their nomenclature.
(1) As in the case of a simple inorganic compound, first the cation is named and then the anion. A space is left between the name of the cation and the name of the anion.
(2) The complex ion in the compound can be either positively charged or negatively charged. First, identify the metal ion and the ligand/s in the complex ion.
(3) The ligands could be negatively charged, neutral or positively charged (rarely). When naming the ligands, the charge of the ligand is considered.
(i) Neutral ligands have no special ending.
(ii) Some ligands have special names.
Examples:
H2O aqua
NH3 ammine
CO carbonyl
NO nitrosyl
(iii) For negatively charged ligands an “o” is added to their English name.
Examples:
Cl– chlorido
CN– cyanido
NO2– nitrito
OH– hydroxido
SCN– thiocyanato
H– hydrido
O2- oxido
(iv) For positively charged ligands the suffix” ium” is added to their English name.
Example: +NH3-NH2 hydrazinium
(4) When there is more than one ligand of the same type, in order to indicate the number of such ligands, the name of the relevant number is used as a prefix to the name of the ligand. When there are 2, 3, 4, 5 and 6 ligands of the same type, the prefixes di-, tri,- tetrapenta- and hexa- are used respectively.
(5) When several ligand types are present in a complex ion, in naming the ligands they are listed in the alphabetical order (English) of the first letter of the ligand.
Note: The first letter of the prefix used to denote the number of ligands is NOT considered when deciding the alphabetical order.
No space is left between the names of the ligands.
Example: [Fe(CN)2(NH3)4]+
Ligands are named as; tetraamminedicyanido
(6) When writing the name of the complex ion, the ligands are named first and then the metal ion. The oxidation number of the metal ion is given in capital Roman numerals, within parentheses, after the name.
No space is left between the words when writing the name.
Examples:
[Co(NH3)6]3+ hexaamminecobalt(III) ion
[Fe(H2O)6]2+ hexaaquairon(II) ion
[Cu(NH3)4]2+ tetraamminecopper(II) ion
(7) The complex may be positively charged, negatively charged or neutral. Depending on this, the name also changes.
(i) When the complex is positively charged or neutral, the name of thecomplex ends with the name of the metal.
Reminder
The name of the metal is followed by the oxidation number of the metal ion, in capital Roman numerals, within parentheses. No space is left between the name of the metal ion and the oxidation number given in parantheses.
Example:
[Fe(CN)3(NH3)3]
The complex is neutral. Hence, its name is triamminetricyanidoiron(III).
Example:
[Cu(H2O)6 ]2+
The complex is positively charged. Its name is hexaaquacopper(II) ion.
(ii) When the complex ion is negatively charged, the suffix ‘ate’ is added to the end of the name of the metal. Here also the oxidation number of the metal ion should be indicated in capital Roman numerals within parentheses. No space is left between the name of the metal ion and the oxidation number given in parentheses.
Examples:
[CoCl4]2- tetrachloridocobaltate(II) ion
[Co(CN)6]3- hexacyanidocobaltate(III) ion
[CuCl4]2- tetrachloridocuprate(II) ion
[Fe(CN)6]4- hexacyanidoferrate(II) ion
[Fe(CN)6]3- hexacyanidoferrate(III) ion
[Ag(CN)2]– dicyanidoargentate(I) ion
[Cr(Br)6]3- hexabromidochromate(III) ion
The IUPAC name of any compound can be developed by systematically following the rules studied so far.
(8) When writing the name of a coordination compound, a space should be left between the name of the positively charged species and the negatively charged species.
Examples :
• Simple cation and complex anion
K3[Fe(CN)5NO] potassium pentacyanidonitrosylferrate(II)
Na2[ZnCl4] sodium tetrachloridozincate(II)
• Complex cation and simple anion
[Ag(NH3)2]Cl diamminesilver(I) chloride
[Fe(OH)2(H2O)4]Br tetraaquadihydroxidoiron(III) bromide
[CoCl(NH3)5](NO2)2 pentaamminechloridocobalt(III) nitrate
[CoCl(NH3)5](NO2)2 pentaamminechloridocobalt(III) nitrite
1. The positively charged species is written first, followed by the negatively charged species. No space is left between them.
2. The complex part of the compound is always written within square brackets.
3. When the formula of the complex ion is written, the metal should be indicated first and then the ligands. In writing the ligands, the charge on the ligand is NOT considered. The ligands are written in the alphabetical order of the ligating atoms. (i.e. Atom through which the ligand coordinates to the metal ion).
Note: In multiatomic ligands, where possible, it is recommended that the ligating atom is placed first followed by the other atoms in the ligand.
Example : (:OH2) rather than H2O
4. Multi atomic ligands are given in parentheses. The number of each type of ligand is given in Arabic numerals as a subscript on the right hand side immediately after the symbol of the ligand. If parentheses are present, this number is written as a subscript on the right hand side without leaving a space, just outside the parentheses.
5. The formula of a complex ion should be written within square brackets. If the complex ion has a charge, it should be indicated outside the square bracket as a superscript on the right side. The numerical value should be given first followed by the sign of the charge.
Note: No space is left between the formulae of the ligands or between the formulae of the ligands and symbol of the metal ion.
Example 1
Write the chemical formula of pentacyanidonitrosylferrate(II) ion.
Step 1: Write the symbol of the metal.
Fe
Step 2: Decide on the order of the ligands. Include the number of ligands of each type when writing their symbols/formulae. Then write the symbols / formulae of the ligands after the symbol of the metal. The ligands are CN– (ligating atom is C) and NO (ligating atom is nitrogen) hence, the order is CN– followed by NO. Both ligands are multi-atomic. Hence, their formulae are placed within parentheses. The presence of five CN– ligands are indicated.
Fe(CN)5(NO)
Step 3: Place the symbol of the metal ion and the formulae of the ligands within square brackets and show charge of complex.
Oxidation number of metal is +II. Overall charge of the complex ion is + 2 + (-5) = -3
Chemical formula is [Fe(CN)5NO]3-
Example 2
Write the chemical formula of pentaamminechloridocobalt(III) ion.
Step 1: Write the symbol of the metal.
Co.
Step 2: Decide on the order of the ligands. Include the number of ligands of each type when writing their symbol/formula. Then write the symbols/ formulae of the ligands after the symbol of the metal.
Ligands are Cl– (ligating atom is Cl) and NH3 (ligating atom is N). Considering the alphabetical order of the ligating atoms, Cl– is written first followed by NH3. The NH3 ligand is multi-atomic and hence the formula is placed within parentheses. The presence of five NH3 ligands is indicated.
CoCl(NH3)5
Step 3: Place the symbol of the metal and the formulae of the ligands within square brackets and show charge of complex.Oxidation state of the metal ion is +III. Therefore, overall charge of the complex is +3 + (-1) = +2
Chemical formula is; [CoCl(NH3)5]2+
Example 3
Write the chemical formula of pentaamminechloridocobalt(III) bromide.
As shown in Example 2, the chemical formula of the complex ion is;
[CoCl(NH3)5]2+.
Two Br– ions are required to neutralize the charge on this complex ion. Therefore, the formula is; [CoCl(NH3)5]Br2
• Almost all cations form atoms in a flame test. The colour of the flame in the flame test is associated with elements possessing low energy gaps.
• The precipitates of the cations having d7, d8, d9 and d10 electronic configurations are soluble in excess ammonia and form the respective stable complex ions.
M2+(aq) + X2-(aq) → MX(s)
(d7) [Co(NH3)6]2+ – Yellow brown
(d8) [Ni(NH3)6]2+ – Deep blue
(d9) [Cu(NH3)4]2+ – deep blue
(d10) [Zn(NH3)4]2+ – colourless
(d10) [Ag(NH3)2]+ – colourless
(d10) [Cd(NH3)4]2+ – colourless
• Ammonium salts give ammonia gas with solutions of alkali(NaOH, KOH, Ca(OH)2 etc).
eg. NH4Cl(s) + NaOH(aq) → NH3(g) + Na+(aq) + Cl–(aq) + H2O(l)
The evolved NH3 gas can be tested with Nessler’s reagent or moist red litmus paper.
NH3(g) + Nerssler’s reagent → brown precipitate/colouration
• Qualitative analysis of a mixture of cations involves the separation of them to five groups. The scheme of qualitative analysis is based on the principle of selective precipitation. The precipitation of cations from a solution one at a time is called selective precipitation.
Group I
• Cold, excess dilute HCl is added to a solution containing a mixture of cations. Only Ag+, Pb2+ and Hg2 2+ will be precipitated as insoluble chlorides (AgCl, PbCl2, Hg2Cl2).
Group II
• After the separation of the insoluble chlorides in Group I, the filtrate is still acidic. When H2S is passed through the solution, only insoluble sulphides get precipitated.
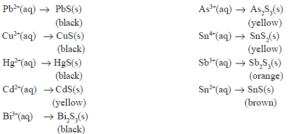 • The concentration of sulphide ion is relatively low because of the higher concentration of H+ ions. Other cations such as Mn2+ , Zn2+ , Ni2+ and Co2+ with higher Ksp values of their respective sulphides will remain in the solution.
• The concentration of sulphide ion is relatively low because of the higher concentration of H+ ions. Other cations such as Mn2+ , Zn2+ , Ni2+ and Co2+ with higher Ksp values of their respective sulphides will remain in the solution.
Group III
• The filtrate from Group II is boiled for a few minutes to expel all the dissolved H2S. Then boil the filtrate for a few minutes with conc.HNO3 to oxidise Fe2+ to Fe3+ . The solution is treated with NH4Cl and NH4OH.
Fe3+ (aq) → Fe(OH)3(s) (reddish brown)
Al3+ (aq) → Al(OH)3(s) (white gelatinous)
Cr3+ (aq) → Cr(OH)3(s) (green)
Group IV
• The filtrate from Group III contains OH– ions and is basic. H2S is passed through this solution in the presence of OH– ions. Then H+ ions produced from H2S are neutralised by hydroxyl ions.
• The above equilibrium shifts to the right and the concentration of S2- ions increases
Group V
• Boils off H2S from Group IV filtrate and add a little amount of NH4Cl and NH4OH in excess. Heat the solution, then add (NH4)2CO3 solution. Here Ca2+, Sr2+ and Ba2+ ions are precipitated as carbonates.
| CHEMICAL TEST FOR | TEST METHOD | OBSERVATIONS | TEST CHEMISTRY |
Chemical test for Carbonate ion CO32– or hydrogencarbonate HCO3– ion
Acid is added to the solid carbonate in a test tube. You could also collect a sample of gas from a heated carbonate, i.e. the solid is where the liquid is in the left hand test tube.
|
(i) Add any dilute strong acid to the suspected solid carbonate – if colourless gas given off, test with limewater.(ii) Effect of fairly strong heating and testing for any carbon dioxide given off.
Test (ii) will distinguish sodium hydrogencarbonate (NaHCO3 readily decomposes – ‘baking powder’) from anhydrous sodium carbonate (Na2CO3, thermally very stable). |
(i) Fizzing – colourless gaswhich turns limewater milky – cloudy fine white precipitate (ii) There might be colour changes in the solid, but you need to collect a sample of gas from just above the heated solid to see it gives a white precipitate with limewater.
Apart from hydrated sodium carbonate, sodium hydrogencarbonate is one of the few common carbonates to give off water on heating and condenses on side of test tube, but basic carbonates will also give off H2O as well as CO2. |
(i) To identify any carbonate/hydrogencarbonate + acid ==> salt + water + carbon dioxide, then white precipitate with limewater. The ionic equations are for carbonate …CO32–(s) + 2H+(aq) ==> H2O(l) + CO2(g)
and for the hydrogencarbonate … HCO3–(s) + H+(aq) ==> H2O(l) + CO2(g) (ii) The thermal decomposition equations are for carbonates MCO3(s) ==>MO(s) + CO2(g) e.g. M = Mg, Zn, CuO and note that some give clear colour changes in the solid which might be useful to identify the metal and for sodium hydrogencarbonate … 2NaHCO3(s) ==> Na2CO3(s) + H2O(l) + CO2(g) |
| Sulphate ion or sulphate(VI) ion SO42–[sulfate, sulfate(VI)] chemical testIf the solution also contains the chloride ion, you test with barium ions 1st, filter off any barium sulphate precipitate and then test for chloride ion. This is because silver sulphate is also ~insoluble. | (i) To a solution of the suspected sulfate add dilute hydrochloric and a few drops of barium chloride/ nitrate solution.(ii) Add lead(II) nitrate solution. | (i) A white precipitateof barium sulfate.(ii) A white precipitate of lead(II) sulphate.
|
(i) Ba2+(aq) + SO42–(aq) ==> BaSO4(s)Any soluble barium salt + any soluble sulphate forms a white dense barium sulphate precipitate.
(ii) Pb2+(aq) + SO42–(aq) ==> PbSO4(s) Neither white precipitate is soluble in excess hydrochloric acid. |
| Sulphite ion or sulphate(IV) ion SO32–[sulfite, sulfate(IV)] chemical testTest (iii) is easily unreliable, the sulphite ion is oxidised by air (dissolved oxygen) to give the sulphate ion, so you will lucky to obtain a clear solution after adding excess acid. | (i) Add dilute hydrochloric acid to the suspected sulfite.(ii) Test any gas evolved with fresh potassium dichromate(VI) paper.
(iii) Add barium chloride or barium nitrate solution. |
(i) Acrid choking sulfur dioxide gas formed.(ii) The dichromate paper turns fromorange to green.
(iii) A white ppt. of barium sulphite which dissolves in excess hydrochloric acid to give a clear colourless solution. |
(i) To identify any sulphite salt + hydrochloric acid ==> chloride salt + sulphur dioxide.(ii) The sulphur dioxide reduces the dichromate(VI) to chromium(III). Note: sulphites do not give ppt. with acidified barium chloride/nitrate because sulphites dissolve in acids.
(iii) Ba2+(aq) + SO32–(aq) ==> BaSO3(s) BaSO3(s) + 2HCl(aq) ==> BaCl2(aq) + H2O(l) + SO2(aq) |
| Sulphide ion S2– (sulfide) chemical testIn test (ii) dangerous hydrogen sulphide (hydrogen sulfide) is formed. | (i) If soluble, add a few drops lead(II) ethanoate solution.(ii) If solid, add dil. HCl(aq) acid, test smelly gas with damp lead(II) ethanoate paper (old name lead acetate). | (i) Black precipitateof lead sulphide.(ii) Rotten egg smell of hydrogen sulphide and the H2S gas turns lead(II) ethanoate paper black. | (i) Pb2+(aq) + S2–(aq) => PbS(s) (ii) MS(s) + 2H+(aq) => M2+(aq) + H2S(g) (e.g. M = Pb, Fe, Cu, Ni etc.) Then reaction (i) above occurs on the lead(II) ethanoate paper (old name lead acetate). |
| Chloride ion chemical testCl–
If the solution also contains the sulphate ion, you test with barium ions 1st, filter off any barium sulphate precipitate and then test for chloride ion. This is because silver sulphate is also ~insoluble, so the two precipitates of silver sulfate and silver chloride could not be distinguished |
(i) If the chloride is soluble, add dilute nitric acid and silver nitrate solution. The silver nitrate is acidified with dilute nitric acid to prevent the precipitation of other non–halide silver salts.(ii) If insoluble salt, add conc. sulphuric acid, warm if necessary then test gas as for HCl.
(iii) Add lead(II) nitrate solution. Not a very specific test – test (i) is best. |
(i) white precipitateof silver chloride soluble in dilute ammonia.(ii) You get nasty fumes of hydrogen chloride which turn blue litmus red and give a white precipitate with silver nitrate solution.
(iii) A white ppt. of lead(II) chloride is formed. |
(i) Ag+(aq) + Cl–(aq) ==> AgCl(s)Any soluble silver salt + any soluble chloride gives a white silver chloride precipitate, that darkens in light.
(ii) Cl–(s) + H2SO4(l) ==> HSO4–(s) + HCl(g) , then Ag+(aq) + Cl–(aq) ==> AgCl(s) (iii) Pb2+(aq) + 2Cl–(aq) ==> PbCl2(s) |
| Bromide ion chemical testBr– | (i) If bromide soluble, add dilute nitric acid and silver nitrate solution. The silver nitrate is acidified with dilute nitric acid to prevent the precipitation of other non–halide silver salts.(ii) If insoluble salt, add conc. sulphuric acid, warm if necessary.
(iii) Add lead(II) nitrate solution. Not a very specific test – test (i) is best. |
(i) Cream precipitate of silver bromide, only soluble in concentrated ammonia.(ii) Orange vapour of bromine and pungent fumes of SO2, test for sulphur dioxide.
(iii) A white ppt. of lead(II) bromide is formed. |
(i) Ag+(aq) + Br–(aq) ==> AgBr(s)Any soluble silver salt + any soluble bromide gives a cream silver bromide precipitate.
(ii) The bromide ion is oxidised to bromine and the sulphuric acid is reduced to sulphur dioxide. (iii) Pb2+(aq) + 2Br–(aq) ==> PbBr2(s) |
| Fluoride Ion chemical testF–
Fluoride and hydrogen fluoride gas are harmful, irritating and corrosive substances. |
(i) If the suspected fluoride is soluble add dilute nitric acid and silver nitrate solution.(ii) You can warm a solid fluoride with conc. sulphuric acid and hold in the fumes (ONLY!) a glass rod with a drop of water on the end. | (i) There is NO precipitate!(ii) Look for etching effects on the surface of the glass rod. | (i) Silver fluoride, AgF, is moderately soluble so this test proves little except that it isn’t chloride, bromide and iodide!(ii) Hydrogen fluoride gas is produced by displacement
F– + H2SO4 ==> HSO4– + HF which reacts with the glass silica to form silicic acid, silicon oxyfluoride, silicon fluoride. The chemistry is messy and complex BUT the glass rod is clearly etched. |
|
Iodide ion chemical test I– |
(i) If iodide soluble, add dilute nitric acid and silver nitrate solution. The silver nitrate is acidified with dilute nitric acid to prevent the precipitation of other non–halide silver salts.(ii) If insoluble salt can heat with conc. sulphuric acid, (ii) get purple fumes of iodine and very smelly hydrogen sulphide.
(iii) If iodide soluble, add lead(II) nitrate solution. |
(i) Yellow precipitate of silver iodide insoluble in concentrated ammonia.(ii) purple vapourand rotten egg smell!
(iii) Yellow precipitate of lead(II) iodide. Not too definitive –Test (i) best. |
(i) Ag+(aq) + I–(aq) ==> AgI(s)any soluble silver salt + any soluble iodide ==> yellow silver iodide precipitate,
(ii) iodide ion is oxidised to iodine and the sulphuric acid is reduced to ‘rotten eggs’ smelly hydrogen sulphide, (iii) insoluble lead(II) iodide formed Pb2+(aq) + 2I–(aq) ==> PbI2(s) |
| Nitrate ion or nitrate(V) ion NO3–chemical test | (i) Boil the suspected nitrate with sodium hydroxide solution and fine aluminium powder (Devarda’s Alloy) or aluminium foil.(ii) Add iron(ii) sulphate solution and then conc. sulphuric acid (the ‘brown ring‘test)
(iii) Strongly heating nitrates of M2+ salts. |
(i) the fumes contain ammonia, which turns redlitmus blue, (ii) Where the liquids meet a brown ringforms
(iii) Nasty brown gas (beware!) ofnitrogen (IV) oxide (nitrogen dioxide) |
(i) The aluminium powder is a powerful reducing agent and converts the nitrate ion, NO3–, into ammonia gas, NH3(ii) NO complex of iron(II) formed
(iii) a general thermal decomposition equation for this reaction is 2M(NO3)2(s) ==> 2MO(s) + 4NO2(g) + O2(g) where M = Pb, Zn, Mg, Cu etc. |
| Nitrite ion or nitrate(III) ion NO2–chemical test | (i) in acid solution it decomposes to give colourless NO gas which rapidly oxidises to nasty brown fumes of NO2, (ii) it decolourises (purple ==> colourless) acidified potassium manganate(VII), (iii) it liberates iodine from acidified potassium iodide solution, (iv) forms ammonia with hot Al powder–foil/NaOH(aq) and gives ‘brown ring’ test | ||
| Alkali:Hydroxide ion chemical test i.e. a soluble base (alkali) which forms the OH– ion in water (note: to completely identify alkalis you need to test for the cation e.g. sodium for NaOH etc.) | (i)Litmus or universal indicator or pH meter.(ii) Add a little of an ammonium salt. | (i) It turns litmusblue, variety of colours univ. ind. dark green – violet for weak – strong.(ii) If strongly alkaline ammonia should be released, | (i) A pH meter gives a value of more than 7, the higher the pH number the stronger the alkali, the higher the OH–concentration, (ii) ammonia gas is evolved:(ii) Ammonia released from the salt.
NH4+(aq) + OH–(aq) ==> NH3(g) + H2O(l) |
| Chromate(VI) ion chemical testCrO42– (yellow)
These tests are not very definitive, but collectively they are a good ‘pointer’! |
(i) Add dilute sulphuric acid.(ii) Add barium chloride/nitrate solution.
(iii) Add lead(II) nitrate solution. |
(i) The yellowsolution turns orangeas the dichromate(VI) ion is formed.(ii) A yellow precipitate of barium chromate(VI) is formed.
(iii) A yellow precipitate of lead(II) chromate(VI) is formed. ‘lead chromate‘ |
(i) CrO42–(aq) + 2H+(aq) ==> Cr2O72–(aq)(ii) Ba2+(aq) + CrO42–(aq) ==> BaCrO4(s)
(iii) Pb2+(aq) + CrO42–(aq) ==> PbCrO4(s) |
S2O32-(aq) + dil.HCl → SO2(g) + S(s)
S2O32-(aq) +AgNO3(aq) → Ag2S2O3 (aq) →Δ Ag2S (black precipitate)
S2O32-(aq) +Pb(NO3)2(aq) → PbS2O3 (aq)(white) →Δ Ag2S(black)
• To a solution of add conc. HNO3, excess of ammonium molybdate and warm. A yellow precipitate will be formed.
• The portion of the universe selected for study is called the system.
• Rest of the universe other than the portion selected for study is called the environment.
• The surface that separates the system and the environment is called the boundary.
• Systems where there is an exchange of energy, matter and work across the boundary are called open systems.
• Systems where only energy and work can exchange across the boundary are called closed systems. (i.e. Boundary is impermeable to matter.)
• Systems where there is no exchange of energy, matter or work across the boundary are called isolated systems.
• The properties that depend upon the amount of matter are named extensive properties. Examples- mass, volume, heat capacity
• The properties that are independent of the amount of matter are named intensive properties. Examples : temperature, pressure, density, viscosity, molar volume, molar heat capacity
• Description of the temperature, pressure and composition of the system is called the state of the system. This information is specific for a particular system.
• The properties with specific values for the state in which a system exits are called state functions. These properties do not depend on the history of the system.
• The change in a function of state depends only on its initial state and final state. It is independent of the route followed.
• Volume, temperature, density, refractive index, enthalpy, entropy, etc. are examples for functions of state.
• The quantity of heat supplied to a system or given out from a system under the condition of constant pressure is called the enthalpy change (∆H). This is a thermodynamic property and a function of state.
• The enthalpy change ( ∆H ) associated with a reaction is given by the difference in enthalpy of the products and reactants
ΔH = ΔHproducts – ΔHreactants
Enthalpy change associated with a reaction ; if ∆H < 0 the reaction is exothermic and if ∆H > 0 the reaction is endothermic.
• According to IUPAC convention, enthalpy changes are reported for unit extent of reaction in kJ mol-1.
Standard enthalpy of sublimation (ΔH°sub )
It is the change in enthalpy that occurs when a mole of a solid element or a mole of a solid compound at the standard state is converted completely into a gas at its standard state.
Ca(s) → Ca(g) ΔH°sub = 193kJmol-1
Standard enthalpy of evaporation (ΔH°vap )
It is the change in enthalpy that takes place when a mole of a liquid compound or element at the standard state is converted into a mole of a gaseous compound/ element at its standard state.
Br2 (l) → Br2 (g) ΔH°vap =30.91 kJmol-1
Standard enthalpy of fusion (ΔH°fus )
It is the change in enthalpy that takes place when a mole of a solid compound or element at the standard state is converted into a mole of liquid compound/element at standard state.
Al(s) → Al(l) ΔH°fus=10.7 kJmol-1
Standard enthalpy of atomization ( ΔH°at )
It is the change in enthalpy that takes place when an element or a compound at the standard state is converted into a mole of gaseous atoms at the standard state.
½Cl2(g) → Cl(g) ΔH°at= 121 kJmol-1
Standard enthalpy of first ionization ( ΔH°IE1 )
It is the change in enthalpy that takes place when a mole of gaseous uni-positive ions are formed by removing an electron from each atom that is most weakly bonded to the nucleus from a mole of gaseous atoms of an element at standard state.
Na(g) → Na+(g) + e ΔH°IE1 =496 kJmol-1
Standard enthalpy of electron affinity (or electron gain) (ΔH°EA )
It is the change in enthalpy that takes place when a mole of uni- negative ions are formed in the gaseous state when electrons are given to a mole of atom in the gaseous state under standard state.
Cl(g) + e → Cl¯(g) ΔH°EA = -349 kJmol-1
Standard lattice enthalpy of an ionic compound ( ΔH°L )
It is the change in enthalpy that takes place when one mole of an ionic compound in the solid state is formed from gaseous positive ionsand negative ions at the standard state.
Na+(g)+ Cl– (g)→ NaCl(s) ΔH°L =- 788 kJmol-1
Born – Haber cycle
The thermochemical cycle that is developed to find the enthalpy change of lattice formation of an ionic compound is called the Born-Haber cycle.

• Entropy of a system is a measure of the randomness of the system.
• Entropy is a function of state and it depends only on the initial and final state of the system and is independent of the path of the change.
• Entropy is also a factor affecting both chemical and physical changes.
• Spontaneous changes in an isolated system takes place with an increase in entropy.
• As the entropy related to a certain system is a function of state, the change in entropy can be calculated by subtracting the initial value of entropy from the final value of entropy.
∆S = S final – S initial
• For a chemical reaction, ∆S = S products – S reactants
• If this change is measured under the standard conditions ∆S θ = Sθ products – Sθ reactants
• The total influence of ∆Η and ∆S on a change is given by the Gibbs energy change which is ∆G. The relationship among these at constant temperature (T) is as follows.
∆G = ∆H – T∆S
At a constant temperature and pressure; for a spontaneous reaction ∆G < 0 for a reaction which is not spontaneous ∆G > 0 for a reaction at equilibrium ∆G = 0
Spontaneity of a system with a constant entropy (∆S = 0) is decided by ∆H and the spontaneity of a change that takes place under a constant enthalpy (∆H = 0) is decided by ∆S.
Properties of Gases
Properties of Liquids
Thus, liquids can be poured and assume the shape of their containers
Properties of Solids
Due to the strong intermolecular forces between neighboring molecules, solids are rigid
Note