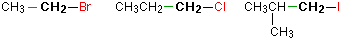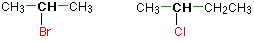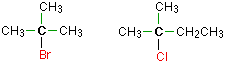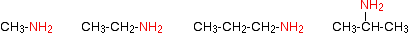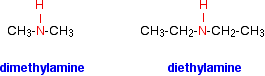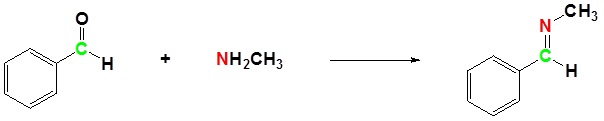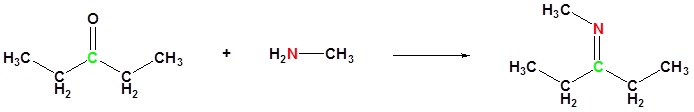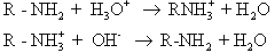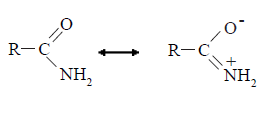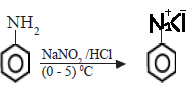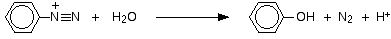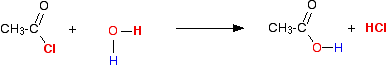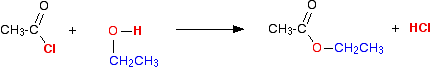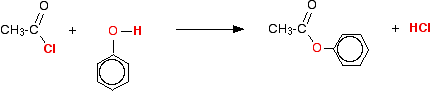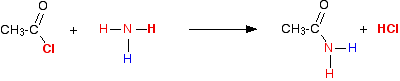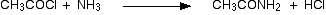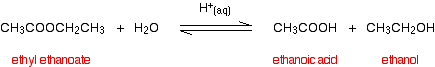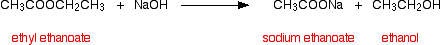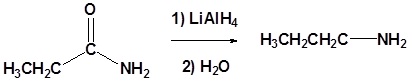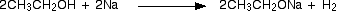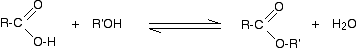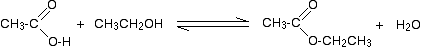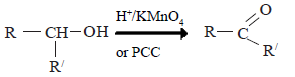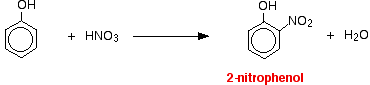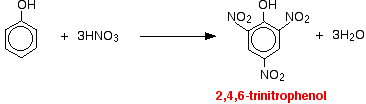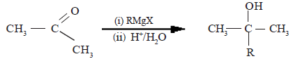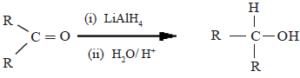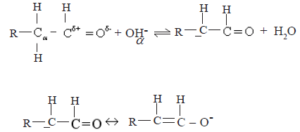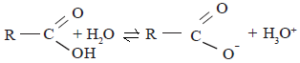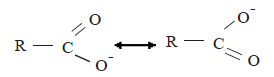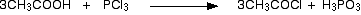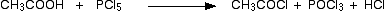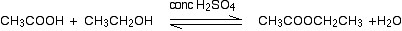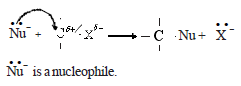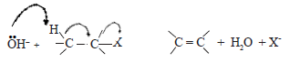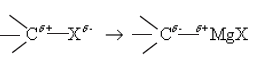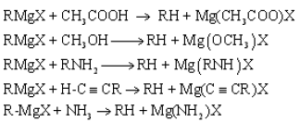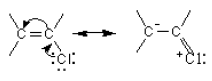• Amines can be defined as compounds where alkyl or aryl groups are attached to nitrogen in place of hydrogen atoms in ammonia.
Amines are classified as primary, secondary and tertiary. Unlike the alkyl halides and alcohols, the amines are classified according to the number of alkyl or aryl groups attached to the nitrogen atom.
The compounds in which an alkyl or an aryl group is attached in place of one of the three atoms of hydrogen in ammonia are called primary amines.
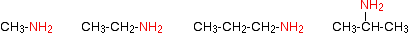
The compounds in which two alkyl or aryl groups are attached in place of two atoms of hydrogen in ammonia are called secondary amines.
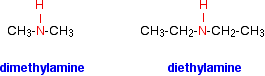
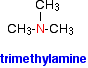
The compounds in which three alkyl or aryl groups are attached in place of the three atoms of hydrogen are called tertiary amines
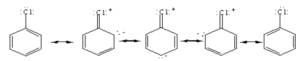
• The compounds in which at least one aryl group is attached to the nitrogen atom are called aryl amines.
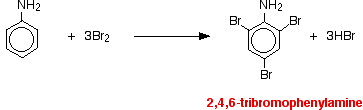
• Aniline readily reacts with bromine water to give a white precipitate as -NH2 group activates benzene ring.
• Amines act as nucleophiles due to the lone pair of electrons on the nitrogen atom. The following are some of the reactions of primary amines with various reagents where the amine acts as a nucleophile.
(i) With acid chlorides

(ii) With aldehydes and ketones
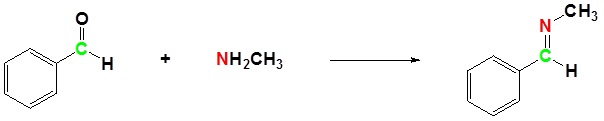
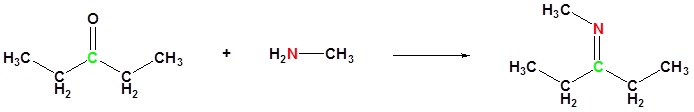
(iii) With alkyl halides
- Ammonia reacts as a nucleophile with alkyl halides to give primary amines in a substitution reaction.
- Yields are often poor as the product, a primary amine, RNH2, is itself a nucleophile and can react with more alkyl halide.
- The result are mixtures containing primary amines, secondary amines, tertiary amines and quaternary ammonium salts.
- This can be avoided if a large excess of ammonia is used.

(iv) With NaNO2/HCl (Nitrous acid)
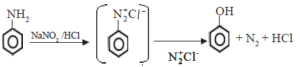
Primary amines react with nitrous acid to form diazonium salts. As alkyl diazonium salts are unstable they rapidly convert to alcohols with the evolution of nitrogen gas.
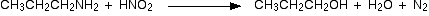
Aromatic diazonium salts formed from aryl amines are more stable particularly at low temperatures.
• Dilute mineral acids convert amines into their salts. These salts react with aqueous hydroxides to regenerate the amine.
Hence it is clear that although amines are more basic than water, they are less basic than hydroxide ions.
![]()
• Amines are more basic than alcohols
R-OH + H+ ⇌ ROH2+ Alkyl oxonium ion
R-NH2 + H+ ⇌ RNH3+ Alkyl ammonium ion
As nitrogen is less electronegative than oxygen it has a higher tendency to donate lone pair of electrons. Hence the stability of the alkyl ammonium ion relative to the amine is stronger than the stability of the alkyl oxonium ion relative to the alcohol. The reason for this is that an atom with low electronegativity can bear a positive charge more easily.
• Aliphatic primary amines are more basic than aniline. The reason for the low basicity of aniline is because the lone pair of electrons on the nitrogen of aniline is not easily available to a proton due to it being delocalized on to the aromatic ring by resonance.

• Amides are less basic than amines. It is because the pair of electrons on the nitrogen of the amide group is delocalized on to the carbonyl group by resonance.
![]()
• Aniline reacts with nitrous acid to give phenol.
![]()
Aromatic diazonium salts are more stable than aliphatic diazonium salts. Therefore, when this reaction is carried out at low temperatures, the conversion of the aromatic diazonium salt to the phenol can be slowed down, and the diazonium salt can be isolated.
![]()
Reactions of diazonium salts in which the diazonium group undergoes replacement.
With water
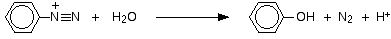
With CuCl,CuBr,KI and HBF4

With CuCN
C6H5N2+ + CuCN → C6H5CN + Cu+ + N2
Diazonium salts can react as electrophiles in electrophilic substitution reactions.
Benzene diazonuim chloride reacts with phenol to give an orange coloured compound, and with β -naphthol to give a red coloured compound.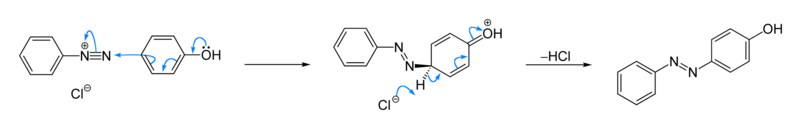

Reactions of acid chlorides
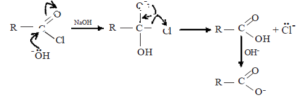
(i) Reaction with sodium hydroxide
Acid chlorides react with NaOH to form the corresponding carboxylic acid which reacts with excess NaOH to form its sodium salt.
![]()
Mechanism of the reaction
![]()
(ii) Reaction with water
Acid chlorides also react with water by a similar mechanism to form the corresponding carboxylic acid.
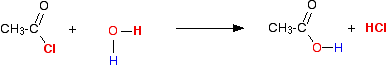
(iii) Reaction with alcohols
Acid chlorides react with alcohols to form esters
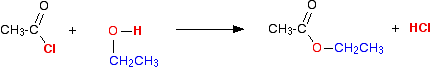
(iv) Reaction with phenol
Acid chlorides react with phenol to form phenyl esters.
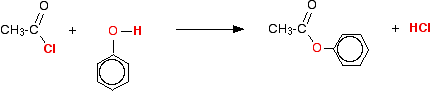
(v) Reaction with NH3
Acid chlorides react with ammonia to form amides
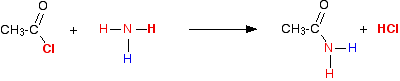
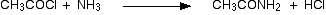
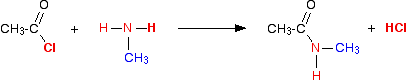
(vi) Reaction with primary amines
Acidchlorides react with amines to form alkyl amines.
Reactions of esters
(i) Esters undergo hydrolysis with dilute acids to form the corresponding carboxylic acid and the alcohol.
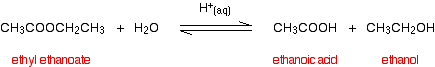
(ii) Esters when reacted with aqueous NaOH form the sodium salt of corresponding carboxylic acid and the alcohol.
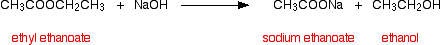
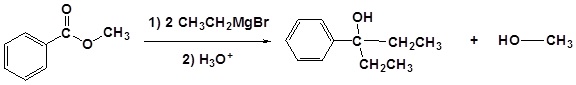
(iii) With Grignard reagent
Esters react with Grignard reagents to give tertiary alcohols. Here, the ester is first converted to a ketone which reacts rapidly with the Grignard reagent again to give the product.
(iv) Reduction by LiAlH4

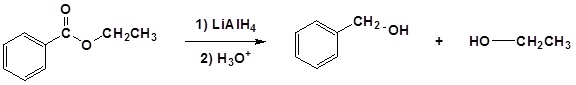
Reactions of amides
(i) With NaOH
When amides are warmed with an aqueous solution of NaOH, NH3 is liberated and the sodium salt of the corresponding carboxylic acid is formed.

(ii) With LiAlH4
Amides are reduced to the corresponding primary amine with LiAlH4
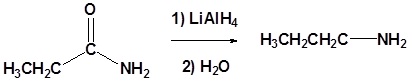
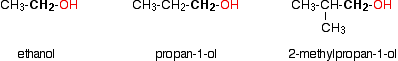
Monohydric alcohols can be classified into three types as primary, secondary and tertiary.
Primary alcohols
Secondary alcohols
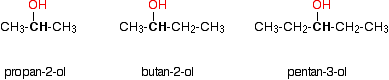
Tertiary alcohols
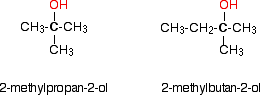
Physical properties
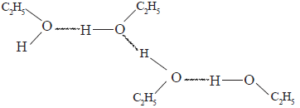
In alcohols the –OH bond is polarized as – R – O δ- -H δ+ . Hence, due to the inter molecular hydrogen bonds formed between alcohol molecules, their boiling points have higher values compared to the alkanes and ether with comparable relative molecular masses. The boiling point increases in going down the series of alcohols.
![]()
The above diagram shows how the inter- molecular hydrogen bonds exist in ethanol. Alcohols which have low relative molecular mass are soluble in water. The solubility of alcohols in water is due to the – OH group which can forms H – bonds with water molecules. The non polar alkyl group in the alcohol molecule is a hindrance to the solubility in water. In going down the homologous series of alcohols the size of the non- polar alkyl group gradually increases relative to the –OH group.
Reactions involving cleavage of the O-H bond
(i) Reaction with sodium
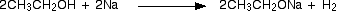
Alcohols behave as acids and react with sodium liberating hydrogen and forming sodium alkoxides. The alkoxide ion is a strong nucleophile and also a strong base.
(ii) Reaction with carboxylic acids (Acylation of alcohols)
Alcohols react with carboxylic acids to form esters. For this esterification reaction, concentrated H2SO4 acid acts as a catalyst.
The esterification reaction is both slow and reversible. The equation for the reaction between an acid RCOOH and an alcohol R’OH (where R and R’ can be the same or different) is:

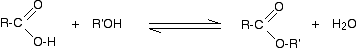
So, for example, if you were making ethyl ethanoate from ethanoic acid and ethanol, the equation would be:

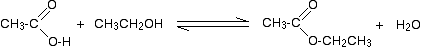
Nucleophilic substitution reactions that take place by the cleavage of C-O bond
(i)Reaction with PCl3 or PCl5
Alcohols react with PCl3 or PCl5 to give alkyl chlorides.
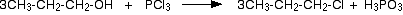
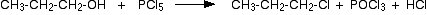
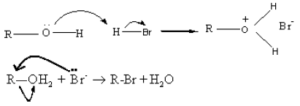
(ii) Reaction with hydrogen halides
![]()
Alcohols under go nucleophilic substitution reaction with HBr to give the corresponding alkyl bromides. Protonation of the O atom converts the -OH group into a better leaving group.
![]() In this reaction Br- ion acts as a nucleophile and the leaving group is H2O.
In this reaction Br- ion acts as a nucleophile and the leaving group is H2O.
(iii) Reaction with anhydrous ZnCl2 and conc. HCl (Lucas test)
In this reaction, R-OH is converted to R-Cl. ZnCl2 is used as a catalyst. Because alkyl halides are insoluble in water, as the raction proceeds the reaction mixture becomes cloudy and turbid. The time taken for the turbidity to appear, after the mixing of reagents, can be used to distinguish between primary, secondary and tertiary alcohols.
Under the provided reaction conditions the above nucleophilic substitution reaction takes place in two steps. Tertiary alcohols form stable intermediate tertiary carbocations and therefore, tertiary alcohols in the presence of the Lucas reagent forms a turbidity in a very short time. Secondary alcohols take longer time and primary alcohols react very slowly.
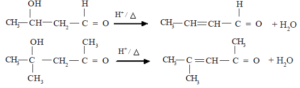
Elimination reactions
Alcohols undergo an elimination reaction when treated with conc. H2SO4 or when heated with alumina to a higher temperature. The reaction in which a molecule of water is eliminated from an alcohol is the dehydration of alcohols. Here, an alkene is formed as the product of the reaction.
![]()
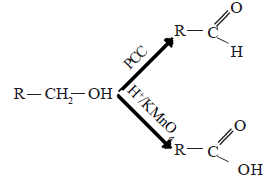
Oxidation of alcohols
The product of oxidation depends on primary, secondary or tertiary nature of the alcohol. Oxidation of alcohols can be carried out with H+/KMnO4 or H+/K2Cr2O2 or H+/CrO3.
(i) Primary alcohols

In the presence of the above mentioned oxidizing agents primary alcohols first give aldehydes. These are further oxidized to carboxylic acids. If pyridinium chlorochromate [C5H5NH+CrO3Cl] is used, the reaction can be stopped at the stage where aldehyde is formed.
![]()
(ii) Secondary alcohols are oxidized to give ketones.
![]()
(iii)Tertiary alcohols
Normally the tertiary alcohols do not undergo oxidation under conditions that primary and secondary alcohols are oxidized.
Aromatic compounds, in which an -OH group is joined directly to a carbon atom of a benzene ring are called phenols. Alcohols and phenols dissociate in aqueous solutions as shown below.
ROH + H2O ⇌ RO– + H3O+
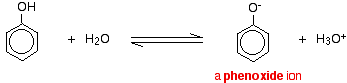
Phenols are more acidic than alcohols. This means that in the above equilibria, the equilibrium point for phenols is more towards the right than alcohols. The reason for this is that the stability of phenate ion relative to phenol is greater than the stability of the alkoxide ion relative to the alcohol. The phenate ion is more stable because its negative charge gets delocalized by resonance. In the alkoxide ion there is no such charge dispersion.
Reactions involving cleavage of the O-H bond
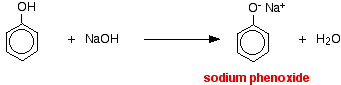
The higher acidity of phenols is confirmed by the following examples too. Although an alcohol reacts with sodium it does not react with NaOH. But phenol reacts with sodium as well as with NaOH. However, phenol is not acidic enough to react with Na2CO3.
2 C6H5OH + 2Na → 2C6H5O– Na++ H2
Non occurance of nucleophilic substitution reactions by breaking C-O bond
Unlike alcohols phenols do not undergo nucleophilic substitution reactions because,
(i) the C-O bond is shorter and stronger due to delocalization of lone pair of electrons
on the oxygen atom into the benzene ring. This can be shown by resonance.

(ii) phenyl cation is unstable.
The effect of the -OH group on the reactivity of the benzene ring in phenol.
• Due to the delocalization of the lone pairs of electrons which were on the oxygen atom with the benzene ring, the ring is rich with electrons. It has become very reactive towards electrophilic reagents. The O-H group of phenol is ortho, para directing. (It is not necessary to explain why the -OH group is ortho, para directing)
• When the electrophilic substitution reactions of phenol are compared with the corresponding reactions of benzene along with the relevant conditions, it is clear that the benzene ring of phenol, had become more reactive towards electrophiles.
Consider the following examples.
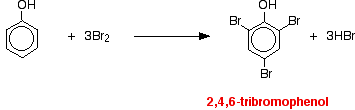
(i) Reacts immediately with bromine water to give a white precipitate of 2,4,6 – tribromophenol.
(ii) For nitration of phenol even dilute HNO3 is sufficiently reactive.
With dilute nitric acid
Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2 nitrophenol and 4-nitrophenol.

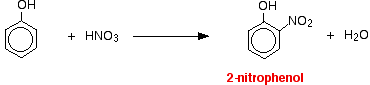

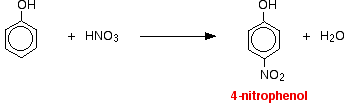
With concentrated nitric acid
With concentrated nitric acid, more nitro groups substitute around the ring to give 2,4,6-trinitrophenol

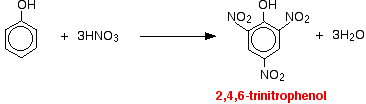
Mechanism of the addition of HCN to aldehydes and ketones.
This reaction is carried out by adding a dilute mineral acid into a mixture of the carbonyl compound and an aqueous solution of sodium cyanide. Here the CN ion acts as the nucleophile.
The mechanism for the addition of HCN to propanone
In the first stage, there is a nucleophilic attack by the cyanide ion on the slightly positive carbon atom.
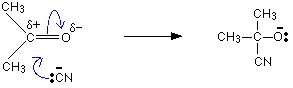
The negative ion formed then picks up a hydrogen ion from somewhere – for example, from a hydrogen cyanide molecule.
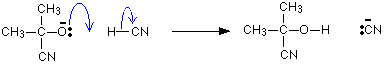
The hydrogen ion could also come from the water or the H3O+ions present in the slightly acidic solution
The mechanism for the addition of HCN to ethanal
As before, the reaction starts with a nucleophilic attack by the cyanide ion on the slightly positive carbon atom.
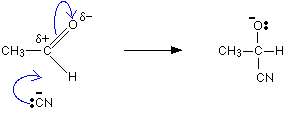
It is completed by the addition of a hydrogen ion from, for example, a hydrogen cyanide molecule.
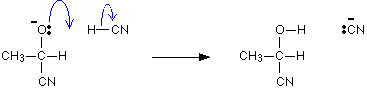
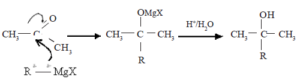
Mechanism of the reaction with Grignard reagent (RMgX)
![]()
![]()
R group of Grignard reagent together with the electron pair of R-Mg bond reacts as a nucleophile with carbonyl carbon.
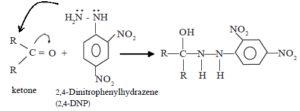
Mechanism of the reaction with Brady’s reagent (2,4-DNP)
(i) Nucleophilic addition
![]()
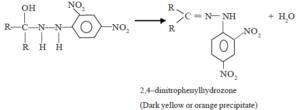
(ii) Dehydration
The above intermediate product undergoes dehydration as soon as it is formed to give the final product, which is 2,4–dinitrophenylhydrozone.
![]()
This reaction is used as a test to identify aldyhydes and ketones.
Reduction
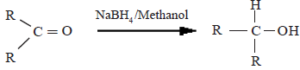
(i) Reduction by LiAlH4 or NaBH4
Here, the aldehydes and ketones get reduced to alcohols.
![]()
![]()
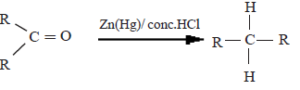
(ii) Reduction by Zn(Hg)/conc. HCl (Clemenson reduction)
Here, the aldehydes and ketones can be reduced to the corresponding hydrocarbons.
![]()
Oxidation of aldehydes
Aldehydes are oxidized to carboxylic acids even by mild oxidizing agents such as Tollen’s reagent and Fehling solution.
(i) Oxidation by Tollen’s reagent
Tollen’s reagent, [Ag(NH3)2]+ is prepared by adding dilute ammonium hydroxide to the precipitate of silver oxide formed by the addition of a few drops of dilute sodium hydroxide to an aqueous solution of silver nitrate.
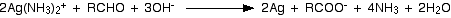
Oxidation by Tollen’s reagent or the silver mirror test is used to distinguish between aldehydes and ketones.
(ii) Oxidation by Fehling solution
A solution of basic cupric tartarate is known as Fehling solution. This is a dark blue aquous solution. When a few drops of an aldehyde are added to this reagent and heated, the blue colour of the solution gradually disappears and a brick red precipitate of cuprous oxide is formed.
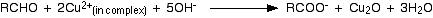
Aldehydes and ketones can be distinguished from each other by Fehling solution.
(iii)Oxidation by oxidizing agents such as acidified potassium dichromate or acidified chromic oxide or acidified potassium permanganate.
Aldehydes get oxidised to carboxylic acids by oxidizing agents such as acidified potassium dichromate or acidified chromic oxide or acidified potassium permanganate.
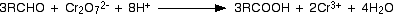
In the presence of aldehydes the pink colour of H+/KMnO4 solution become colourless. The orange colour of H+/Cr2O72- solution turns green. By using these reagents also aldehydes and ketones can be distinguished from each other.
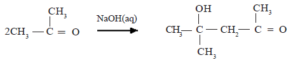
The reactivity of the alpha position of aldehydes and ketones as exemplified by selfcondensation reactions.
• Due to the strong electron withdrawing nature of the carbonyl group, H atoms attached to the carbon atoms directly bound to the carbonyl carbon (the α- H) become acidic. The α- H can be abstracted as a proton by base (eg. OH–).The carbanion so formed is stabilized by resonance as shown below
![]()
The above carbanion attacks the carbon atom of the carbonyl group of an unionized aldehyde molecule as a nucleophile. Hence aldehydes and ketones with α hydrogens undergo self – condensation reactions.


Examples
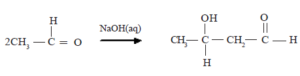
Reaction of acetaldehyde in the presence of aqueous NaOH
![]()
Condensation of acetone
![]()
The addition products obtained above undergo dehydration easily.
Examples :
![]()
• The functional group of the carboxylic acids is the carboxyl group. In the carboxyl group there is a carbonyl group and a hydroxyl group attached to that carbon atom.
• Carboxyl group is a polar functional group. Due to the polarity of C – O and O – H groups it forms intermolecular hydrogen bonds. Relative to the aldehydes and ketones with equal relative molecular masses, the boiling points of carboxylic acids are higher.
• Carboxylic acids of C1 to C4 dissolve well in water. When the number of carbon atoms increases solubility decreases. Aromatic carboxylic acids are water insoluble and exist as solid crystalline substances. Almost all the carboxylic acids are soluble in organic solvents.
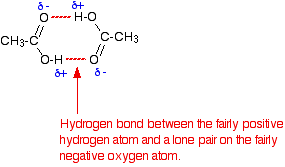
• The ability to form dimeric structures where carboxylic acid molecules are attached by hydrogen bonds as pairs is also a reason for the high boiling points of carboxylic acids.
Existence of carboxylic acids as dimer structures attached by hydrogen bonds is shown below.
Reactions of carboxylic acids
Reactions involving the cleavage of O – H bond
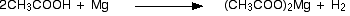
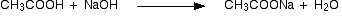
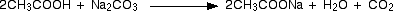
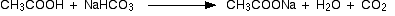
The above compounds react with Na2CO3 in the same way that they react with NaHCO3.
The acidic strengths of alcohols, phenols and carboxylic acids vary as follows.
Alcohols < Phenols< Carboxylic acids
The carboxylic acids attain the following equilibrium in an aqueous solution
![]()
The equilibrium point of the above equilibrium is situated more shifted towards the right side relative to the corresponding equilibrium attained by the phenols. The reason for this is that the stability of the carboxylate ion relative to the carboxylic acid is greater than the stability of the phenoxide ion relative to phenol. The carboxylate ion is a resonance hybrid of the following structures.
![]()
The stability of the carboxylate ion is due to the delocalization of the negative charge between two equivalant electronegative oxygen atoms.
Reactions involving cleavage of the C – O bond
• With PCl3/ PCl5
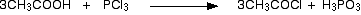
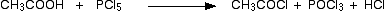
• With alcohols
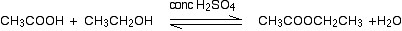
• Reduction of carboxylic acids with LiAlH4
Carboxylic acids give alcohols when reduced with LiAlH4 which is a very strong reducing agent. Carboxylic acids and their derivatives are not reduced by NaBH4.
![]()
Physical state and smell
Haloalkanes are colorless, sweet-smelling liquids. The lower members like methyl chloride, methyl bromide and ethyl chloride are colorless gases while members having very high molecular masses are solids.
Solubility
Haloalkanes are not able to form hydrogen bonds with water and, even though they are polar in nature, they are practically insoluble in water. However, they are soluble in organic solvents like alcohol, ether, benzene, etc.
Density
Chloroalkanes are lighter than water while bromides and alkyl iodides are heavier. With the increase in the size of the alkyl group, the densities go on decreasing in the order of :
fluoride > chloride > bromide > iodide.
Boiling points
The boiling points of alkyl chlorides, bromides and iodides follow the order RI > RBr > RCl where R is an alkyl group. With the increase in the size of halogen, the magnitude of Van der Waals forces increases and, consequently, the boiling points increase. Also, for the same halogen atom, the boiling points of haloalkanes increase with increase in the size of alkyl groups.
Alkyl halides are named as primary, secondary or tertiary depending on the number of H atoms attached to the carbon atom which carries the halogen atom.
primary
secondary
tertiary
Due to the high electronegativity of the halogen atom relative to the carbon atom, the C – X bond is polarized. As a result, there is a deficiency of electrons in that carbon atom. Therefore, nucleophiles attack this position. Nucleophiles are reagents which show a tendency to attack carbon nuclei and which are basic and rich in electrons. A nucleophile will bring a pair of electrons to form a bond with carbon.
Examples- OH– , CN– , R-C≡C– , R-O–
Nucleophilic substitution reactions are characteristic of alkyl halides. Here, the carbon atom forms a new bond with the nuclephile and the halogen atom leaves as a halide ion.
As a nucleophile possesses a pair of electrons available to form a new bond any nucleophile can also act as a base by forming a bond with H+ . Therefore, when an alkyl halide is reacted with a reagent such as :OH– , it can also undergo an elimination reaction by the mechanism shown below.
Here, :OH– instead of reacting as a nucleophile with carbon, reacts as a base and removes a H+ from the carbon atom adjacent to the carbon atom bearing halogen. The hydrogen atoms attached to the carbon atom adjacent to the carbon atom bearing the halogen atoms, have a low acidity due to the polarization of the C-X bond. Thus substitution and elimination are competing reactions in alkyl halides.
Alkyl halides react with Mg in the medium of dry ether to form the Grignard reagent
When an alkyl halide forms a Grignard reagent the polaritiy of the carbon atom originally joined to halogen, changes as shown below.
Due to the polarization of bonds in RMgX, the carbon attached to magnesium acts as a strong nucleophile and a very strong base.
RMgX + H2O → X-Mg-OH + RH
The strong basic character of the Grignard reagent can be shown by the following reactions.
• To explain the nucleophilic substitution reaction of alkyl halides, the time interval between bond breaking and bond making steps can be considered.
• When the breaking of the C-X bond and the formation of the new bond to the nucleophile takes place simultaneously, the nucleophilic substitution reaction of the alkyl halide takes place as a one step reaction.
Accordingly, the one step reaction can be presented as follows:
![]()
• When the formation of the new bond to the nucleophile takes place after the breaking of the C-X bond the nucleophlic substitution reaction of the alkyl halide takes place as a two step reaction.
Accordingly, the reaction that takes place by two steps can be presented as follows.
![]()
The reaction that takes place by two steps goes through an intermediate carbocation. On considering the stability of the carbocation formed, the tertiary alkyl halides (R1, R2, R3 = alkyl) which are able to form a more stable tertiary carbocations undergo nucleophilic substitution in two steps. The primary alkyl halides (R1, R2 = H, R3 = H or alkyl) undergo nucleophilic substitution reactions in one step as they are unable to form a stable intermediate carbocation.
The pathway taken by the secondary alkyl halides (R1 = H, R2, R3 = alkyl) depend on the reaction conditions.
Vinyl and phenyl carbocations are unstable and therefore, they do not react by the two step pathway. They also do not react by the one step path because the C-X bond is stronger than in alkyl halides due to its double bond character. This can be shown by resonance.
![]()
![]()