• Earth is a crucial source of natural capital including essential metals, fuels, and plant
nutrients.
• These earth resources are used in various natural and human processes.
• Earth is also an important repository of wastes enriching soil fertility.
• Unfortunately, recycling process is not uniform around the globe.
• In some locations, waste is accumulated creating hazardous environment. Whereas, some essential nutrients are deprived requiring the addition of synthetic chemicals such as agrochemicals.
• Leaching of hazardous substances from the accumulated wastes contributed to degrade the soil fertility and threatens the existence of different forms of life Sources of soil pollution
Household wastes
• Includes the food waste, human excretes, waste water, garden debris, plastics Agrochemicals
Pesticides
• Pesticide is a substance used for controlling, preventing, destroying, repelling or mitigating any pests. There are two main classes of pesticides used.
Natural pesticides
• Neem (Kohomba) extract is an example for a natural pesticide.
Synthetic pesticides.
• The main types of pesticides used are weedicides and insecticides.
• Weedicides (weed killers) kill plants that would otherwise compete with crop for light and nutrients.
• Insecticides kill insects that would damage the crop.
• Insect pests can reduce the yield in two main ways. They might eat the part of the plants that the farmer wants to harvest. By damaging leaves they reduce photosynthesis which affects the food production.
• Insecticides used against insect pest are of three types (Based on their action).
Three main groups of synthetic insecticides are known.
• Chlorinated hydrocarbons – eg. DDT – (Dichlorodiphenyltrichloroethane).
• Organophosphorus – eg. Melathion.
• Heavy metal salts – eg. Copper dithiocarbomates.
An ideal pesticide should have the following properties
• Should kill only the target pest.
• Should biodegrade easily in the environment or soil water system.
• The pest should not develop any tolerance to the pesticide.
• Should be cheap, abundant and non-toxic to humans.
Problems of using pesticides
• Pesticides can be deposited on our food. They can harm people as well. If the dose is
large enough it could poison us.
• Bio accumulation of persistent pesticides leads to concentration at each level of the
food chains. The concentration at the end of the food chain may reach very high levels.
• They damage the environment. Pesticides often kill harmless or even beneficial species as well as pests.
• Pests can become resistant to pesticides over a period of time. Repeated use of same
pesticide increases the resistance to it through natural selection. Then the pesticide becomes less effective. Natural predators are killed by the pesticide. This may cause a worse outbreak than before.
L.D. value – Lethal dose value LD50.
The chemical dose needed to kill 50% of the population of one species under test is called LD50
Fertilizers
• Fertilizers make plant grow faster because they give the plant essential minerals and
nutrients. eg. NPK
• The most important mineral ions are nitrate, phosphate and potassium. But there are
some trace elements needed in small amounts. NPK content of a fertilizer is expressed as N%, P2O5 %, K2O%.
There are two main types of fertilizers. Each has its own advantages.
• Natural fertilizers are organic matter. They include manure, sewage and sludge.
• Natural fertilizers supply a wide range of nutrients and release them slowly with long lasting effects. They are less harmful to the environment and are suitable for “organic” farming.
• Natural fertilizers are cheap.
• Natural fertilizers can improve the soil structure (texture).
• Natural fertilizers are expensive to transport and to apply and might not have ideal balance of nutrients.
• Artificial fertilizers are inorganic. They contain pure chemicals (eg. NH4NO3) as powders or pellets.
• Artificial fertilizers are fast acting and easy to transport and supply.
• Artificial fertilizers can be used to target particular mineral ions needed and the amount of each mineral supplied can be accurately controlled.
• Artificial fertilizerscan affect the balance of the soil and are more easily washed out of the soil leading to eutrophication of surface water bodies.
• Artificial fertilizers can affect the quality of ground water.
eg. Nitrate content.
Heavy metals
• Used and scrapped metals, used equipments, vehicles
• Heavy metals are leached in to drinking water and contaminate soil
• Uptake of heavy metals through drinking or food can cause numerous health problems
• Heavy metals such as lead can accumulate in body lowering the intelligence e-waste
• The term “e-waste” is used to identify all the waste originated from electronic and
electrical equipments and related accessories including used or outdated computers,
electronic equipments, mobile phones, televisions, sound systems, CFL bulbs, electric
and electronic accessories.
• The impact of e-waste has already witnessed by the developed countries and they are
attempting to mitigate the problem by dumping them to poor countries.
• Rapid technology change, low initial cost, high obsolescence rate have resulted the ewaste to be the fastest growing problem making yesterday’s electronic dream machines to become today’s environmental nightmare.
• The average obsolescence rate for a computer is estimated to be 7 years, and it is 15
years for a television or a refrigerator or a washing machine while a mobile phone has a life span of 1.5 years only.
• Common list hazardous chemicals from e-waste include metallic lead (in batteries, circuit boards, cathode ray tubes in TV), mercury (in thermometers, thermostats, discharge lamps, sensors, relay and switches), cadmium (in batteries, mobile phones), beryllium(in computer, telecommunication equipments, and automotive electronics), arsenic (inlight emitting diodes), polyvinyl chlorides (in computer casings and cables),
polychlorinated biphenyls (in transformers)
• Also form hazardous fumes upon burning the PVC containing e-waste.
3R Systems (Reduce, Reuse and Recycle)
• Reduce, Reuse
Most effetive way to reduce waste is to not create it in the first place. Making a new
product requires a lot of materials and energy: raw materials must be extracted from theearth, and the product must be fabricated and then transported to wherever it will be sold.As a result, reduction and reuse are the most effective ways you can save natural resources,protect the environment and save money.Using as raw materials for the other productions
• Solid wastes can be used to produce various materials.
• Chromium wastes in a tannery effluent can be precipitated as Cr(OH)3 using MgO and is reused in all leather tannery processes.
Using as raw materials for energy generation
• Dry garbage is a fuel. In Sri Lanka about 80% of the garbage is organic material. They
can be converted to obtain energy.
(CHO)n + O2(g) → CO2(g) + H2O(g) + heat
Heat can be used in industries .
• Recycling
Recycling is the process of collecting and processing materials that would otherwise be
thrown away as trash and turning them into new products. Recycling can benefit your community and the environment.
• Many countries have established the recycling of domestic and industrial waste water.Since a large quantity of ground water is used by some industries in Sri Lanka, it is important to encourage them to reuse the water in the same industry. Since the composition of garbage in Sri Lanka is over 80% organic matter, the solid waste can easily be used as a fuel to generate electricity.
• Metals are valuable resources. Instead of burying metal waste it makes sense to collect it and recycle the metals. There are two savings, for instance when scrap iron is collected, melted and re-used, it saves earths reserves of iron ore. In addition the energy required to mine ore, transport it and smelt it is several times greater than the energy required to recycle scrap iron.
• Glass, paper and plastic also can be recycled.
• These materials can be recycled if they are collected and separated at the sources.
Benefits of the recycling process
• Saving in energy
• Saving in natural resources
• Saving in refuse disposal costs
• A source of income for local authorities
• Composting
Fresh plant biomass has a C:N ratio of 100:1. Organic matter is decomposed in solid by
bacteria and fungi (microorganisms) to form humus with a C:N ratio of 10:1. If the C:N ratio of the organic matter in the soil is too high, nitrogen may be the limiting factor in the growth of organisms which decompose organic matter and recycle nutrients. If straw (C:N=80:1) is ploughed in to the soil, a nitrogenous fertilizer is usually applied to lower the C:N ratio.
Composting can be used to reduce the C:N ratio. Storing organic matter in a compost pile with moisture and air allows carbon dioxide and water to escape, while nitrogen is retained as amino acids and proteins of microorganisms. Adding fertilizer to compost increases the population of microorganisms and speeds composting.
• Biogas production
Biogas typically refers to a gas produced by breakdown of organic matters in the absence of oxygen. Organic waste such as dead plants and animal materials, animal feces, and kitchen waste can be converted into a gaseous fuel called biogas. Biogas originates from biogenic material and is a type of bio fuel.
Biogas is produced by the anaerobic digestion or fermentation of biodegradable materials such as biomass, manure, sewage, municipal waste, green wastes, plant material and crops.Biogas comprises primarily methane, and carbon dioxide and may have small amounts of hydrogen sulphide, moisture, etc.
• Incineration
The incineration of waste needs a temperature at which complete combustion of oxidisable material will occur and ash, glass, metal and other materials remain. A temperature of 770-970 0C is used. In many incinerators the heat of combustion of the waste is used to help to maintain the temperature. It is always recommended that the solid wastes from hospitals should be subjected to incineration.
• Polymers are large molecules formed by the union of a large number of repeating units.
eg. n(C2H4) Polymer
Polymers can be classified as natural and synthetic.
• Natural polymers are naturally synthesised in living/bio systems.
eg. Natural rubber, proteins enzymes.
• Synthetic polymers are synthesised by man (man made polymers).
eg. Polythene, polyvinyl chloride, polyesters, Teflon, Bakelite, nylon,urea formaldehyde.
Polymers can be classified in accordance with the method of synthesis.
• Addition polymers
• Condensation polymers
Polymers can be classified based on their thermal properties.
• Thermoplastic polymers :
Can be softened by heating, allow to cool and harden and then re-softened many times. The forces of attraction between chains are weak.
eg. Polythene, PVC, Polystyrene
• Thermosetting polymers:
During the first stage of manufacture, once moulded they set and then cannot be re-softened by heating. Polymer chains are cross linked to form a three dimentional structure.
eg. Bakelite, Urea-formaldehyde
Unsaturated monomers undergo additional polymerization to form addition polymers.

Polythene is a tasteless, odourless, light, non-poisonous, relatively cheap, thermoplastic polymer. It is used to produce packing materials, trash bags, seat covers, bottles,various containers, toys etc.
• Although PVC is a thermoplastic, due to the presence of chlorine, PVC does not catch fire easily. Its attractive forces among the chains are stronger than those in polyethylene and therefore is more harder. A special feature of PVC is that it can be processed with stablisers and fillers making it versatile. PVC is used to make water pipes, gutters, wire insulations, films, floor covers, seat covers, raicoat covers, umbrella materials, etc.
• The monomer is styrene.
• Polystyrene is a transparent substance and a thermoplastic. Made into foam and solidified, it can be used as a insulation and packing material popularly known as ‘Rigifoam’.
• Tetrafluoroethene is the monomer.
• Although it is a thermoplastic due to the presence of halogen it can withstand high temperatures. Therefore, it is used in making fire proof clothing. In addition Teflon is not wettable and therefore, it is used as coatings in non-stick cooking vessels. Being chemically inert, teflon can resist almost all corrosive chemicals, so it is used in valves, seals, gaskets etc. in plants used in chemical industry.
• Rubber is used to make tyres, tubes, mattresses and gloves used in the medical field.
Natural rubber vulcanization
• Presence of cis-polyisoprene chains is the reason for elasticity of rubber. In order to change the elastic nature of rubber as required and to make it stronger, it is heated with 1% – 3% sulphur by weight. This is called vulcanisation. Vulcanisation makes cross links among polyisoprene chains through sulphur bridges and more resilient and tougher.By heating with 25% – 35% sulphur, ebonite is obtained. The vulcanized rubber is not sticky and has superior mechanical properties such as higher elesticity.
Rubber compounding
Natural rubber or other rubber itself may not be a useful material for a particular purpose.But, based on the material mixed, rubber compounding makes it a useful material with desired properties. Material used in rubber compounding can be classified by the function they serve. Main functional cases are given below.
• Elastomers
• Vulcanizing or cross linking agents
• Accelerators
• Activators / retarders
• Process aids
• Softeners and plasticizers
• Reinforces / Fillers
• Age resistors
For example, cover of a conveyor belt is made of the following compounds.
Natural rubber (Elastomer)
Carbon black (Filler)
ZnO (Accelerator)
Stearic acid
Rubber process oil
Resin
N-Cyclohexylbenzothiozole-2-sulfonamide (CBS)
Sulphur (Vulcanizing agent)
The polymers formed by the joining of monomers with elimination of small molecules such as H2O, NH3 or HCl are called condensation polymers.
• Polymers formed by joining of monomers by – CONH – group are known as polyamides.
Nylons are polyamides. One of the commonest is nylon 6,6 which is made by condensation polymerisation between 1, 6 – diaminohexane and hexanedioic acid. The reaction is more effective when acid chloride is used instead of the dicarboxylic acid.

• Nylon is a thermoplastic polymer and it is used to produce fibres which have structural similarities to silk and wool. Despite its structural similarity to wool, nylon lacks the softness and the moisture – absorbing properties of the natural fibre. It is, however, harder wearing and has good ‘wash and wear’ characteristics. Nylon is mainly used to make soft and light clothing. Other uses include tufted carpets, tyre cords, machine gear wheels and bearings, fishing nets and non wetable tent covers. The elasticity and strength of nylon make it ideal for making stockings and tights.
• Polymers with monomers linked by the -COO- (ester) groups are commonly called
polyesters.
Terelene formed by the condensation polymerisation between ethane -1,2-diol(ethylene glycol) and benzene – 1,4 – dicarboxylic acid (Terephthalic acid) is an example for a polyester.

• Terelene is a thermoplastic. It is used as a substitute for natural fibres such as cotton and wool. Being strong, the fibres are used in the manufacture of fibre glass. It is also used in the production of textiles, photographic films and audio tapes.
In the presence conc. H2SO4 phenol and formaldehyde (methanal) react to form a thermosetting polymer know as Bakelite.
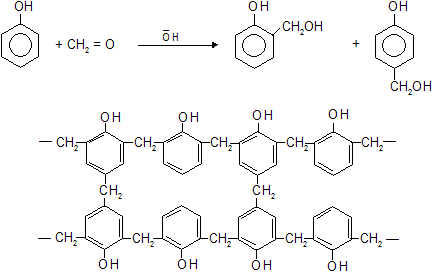
• Bakelite is a cross linked three dimensional polymer. These cross links lead to a very rigid structure because the various groups are not free to twist round and move their positions. Bakelite is used to make heat resistant parts of electric utensils.
In the presence conc. H2SO4 urea and formaldehyde react to form a thermosetting polymer know as urea-formaldehyde.
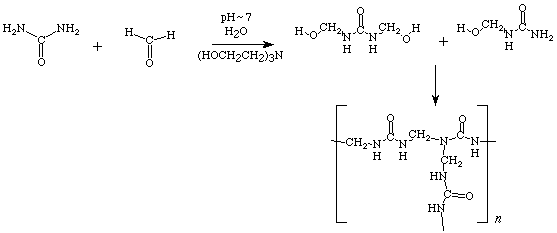
• Urea-formaldehyde is a cross linked three dimensional polymer. Ther are used as thermosetting plastic or adhesives.
• Capital • Technology
• Availability of raw materials • Power (electricity, fuel, etc.)
• Labour • Transport facilities and the market
• Waste management • Controlling the environmental pollution
• Government rules and regulations
• Characteristics of natural resources that can be used as raw materials for an industry
• Occurring as large ores appropriate for long term usage
• Easy access • High purity
• s- block elements do not occur freely in the nature because they are very reactive and they exist as compounds
Rock salt – NaCl
Sea water – NaCl, MgCl2, CaCl2, CaSO4, Ca(HCO3)2, MgSO4
Silvine – KCl
Borax – Na2B4O7.10H2O
Beryl – 3BeO.Al2O3.6SiO2
Magnesite – MgCO3
Dolomite – CaCO3.MgCO3
Limestone,Marble,Oyster shells – CaCO3
Gypsum – CaSO4.2H2O
Fluorspar – CaF2
Apatite – Ca5(PO4)3X or 3Ca3(PO4)2.CaX2 (X = F, Cl,OH)
• Sodium is extracted using electrolysis of molten NaCl. CaCl2 is added to reduce the
melting point of NaCl to 600 °C.
• At the cathode,
Na+(l) + e→ Na(l)
• At the anode,
2Cl–(l) → Cl2(g) + 2e
• Anode and cathode are separated by a circular disc (steel gauze diaphragm) to prevent the reaction between chlorine gas and sodium.
• A large current is passed through the cell, but at a low voltage.
• Sodium vapour lamps
• Molten sodium is used as a coolant in nuclear reactors.
• Solid sodium is used to dry organic solvents like ether and benzene.
• Used in organic synthesis
• Used to synthesise sodamide (NaNH2) which is a strong reducing agent.
The places where salt produced are referred to as saltterns. In Sri Lanka two main saltterns are placed in Puttalam and Hambantota.
Characteristic features of the places where saltterns are established
• Plane land by the sea or lagoon • Less rainfall
• Dry air, more sunlight • Water impervious clay sand
• Sea water is used as the raw material.
• Sea water is pumped into the first tank of the salttern. The sea water is evaporated by sunlight. When the concentration of sea water increases CaCO3 gets precipitated in the first tank of the salttern. CaCO3 precipitate is allowed to settle down.
• Remaining solution is transferred into the second tank of the salttern and evaporated by sunlight. When the concentration further increases CaSO4 gets precipitated.
• Remaining solution is transferred into the third tank of the salttern and evaporated. When the concentration further increases NaCl gets precipitated. NaCl is removed from the third tank of the salttern. This NaCl contains Ca2+, Mg2+ and SO42- as impurities.
• Pure NaCl is not hygroscopic. But NaCl having the impurities is hygroscopic. Sodium chloride collected from the third tank is stored outside for nearly six months. During this storage period NaCl is almost purified as Ca2+ and Mg2+ salts absorb water from air and become solution while NaCl remains as a solid.
• Iodized salt is produced by mixing with KIO3.
• Cooking
• Food preservative (Maldivian fish, Pickle)
• Manufacture of Na metal, Na2CO3, NaHCO3 and NaOH
• Saline
• To reduce the melting point of ice
• As Mg2+ and Br- concentrations are high in bittern soultion it can be used to produce Mg and Br2.
• Sodium hydroxide is commercially produced by the electrolysis of aqueous sodium chloride in chloro-alkali cells.
• There are three types of chloro alkali cells
• Mercury cell • Diaphragm cell • Membrane cell
• The membrane cell is very similar to the diaphragm cell and the same reactions occur. The main difference is that two electrodes of membrane cell are separated by an ion selective membrane rather than by a diaphragm.
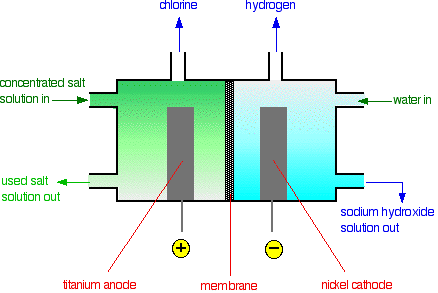
• The advantage of using membrane cell is that the NaOH that is produced is very pure and also uses less electricity, lowest environmental impact.
• The half reactions are
2Cl–(aq) → Cl2(g) + 2e (at anode)
2H2O(l) + 2e → 2OH–(aq) + H2(g) (at cathode)
• The overal reaction is
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
• The anode is made of titanium and cathode is made of nickel.
• The anodic and cathodic compartments are separated by a polymer cation-exchange membrane.
• The membrane can exchange cations and hence permits Na+ ions to migrate from anodic compartment to cathodic compartment.
• The flow of cations maintain electro-neutrality in the two compartments because, during electrolysis, charge is removed at the anode and supplied at the cathode.
• OH– would react with Cl2, and spoil the process. But migration of OH– is suppressed because the membrane does not exchange anions.
• NaOH solution is partially evaporated and allowed to cool.
• Chlorine is produced as a by-product.
• Use in the laboratory as a convenient strong base
• Absorb carbon dioxide and other acidic gases
• Manufacture of soap, paper, atificial silk and dye stuffs
• Treatment of effluents for removal of heavy metals (as hydroxides) and of acidity
• Used directly or in combination form as a bleach for textiles, wood, and paper pulps.
• Disinfection of drinking water
• One quarter of the hydrochloric acid produced in U.K. is synthesized from chlorine and hydrogen.
• Used in recovery of tin, titanium and magnesium from scrap.
• Used to produce chlorinated rubbers, insecticides, dyes and drugs.
• Used in the manufacture of polymeric materials such as polyvinyl chloride
Oils, fats or their fatty acids and inorganic water soluble bases (NaOH, KOH) are used as raw materials.
• The industrial soap making involves four basic steps.
• Step 1 – Saponification
The saponification process involves the mixing of tallow (animal fat), coconut oil or vegetable oil with sodium hydroxide and the application of heat. The process results in formation of soap, which is a salt of long chain carboxylic acid.

• Step 2 – Removal of glycerin
Glycerin is more valuable than soap, and hence most of it is removed for its uses in more expensive cosmetic products. Some of the glycerin is left in soap to make it soft and smooth.
• Step 3 – Soap purification
In the soap purification stage any remaining sodium hydroxide is neutralized with a weak acid like citric acid and two thirds of the remaining water is removed to obtain pure soap.
• Step 4 – Finishing
The final stage of industrial soap manufacturing process, finishing stage involves mixing of additives such as colours, preservatives and perfume into soap, which is then shapedinto bars for sale.
• Instead of NaOH, KOH could be used. The soap manufactured using KOH is softer on skin. Therefore KOH is used mainly in the manufacture of baby soap.
• The percentage of RCOO–Na+ in the soap is referred as total fatty matter(TFM) value.
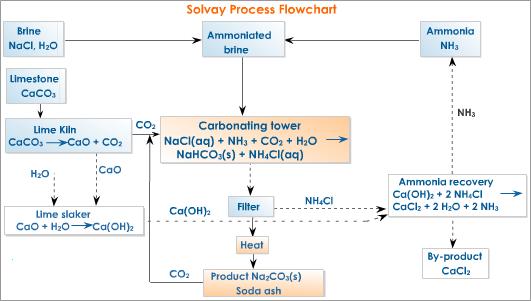
• Brine (concentrated NaCl solution), limestone and ammonia (manufactured by Haber process) are used as raw materials.
• NH3 gas is dissolved in brine. This reaction is exothermic. Therefore low temperatures are favoured.
• Counter current principle is used to achieve higher efficiency of dissolving.
• Brine saturated with NH3 is allowed to react with CO2(g) which is obtained by heating limestone. The reaction is exothermic. Therefore, low temperatures are favoured.
• Again the counter current principle is used for achieving higher efficiency.
Here, following reversible reactions take place.
NH3(aq) + H2O(l) → NH4+ (aq) + OH–(aq)
OH– (aq) + CO2 (aq) → HCO3–(aq)
• As OH– is removed by the second reaction more and more OH– ions are formed by the first reaction.
• When HCO3– concentration increases NaHCO3 crystallizes.
Na+(aq) + HCO3–(aq) → NaHCO3(s)
• Low temperature is maintained to facilitate the separation of solid NaHCO3
• NaHCO3(s) is isolated and heated. CO2 formed is used again.
2NaHCO3(s) →Δ Na2CO3(s) + CO2(g) + H2O(g)
• Net reaction for the formation of NaHCO3 is
NaCl (aq) + NH3(aq) + CO2(aq) + H2O(l) → NaHCO3(s) + NH4Cl(aq)
• The NH4Cl and lime are used to regenerate NH3. This NH3 is used again.
CaO(s) + 2NH4Cl(aq) → CaCl2(aq) + 2NH3(aq) + H2O(l) or
Ca(OH)2(s) + NH4Cl(aq) → CaCl2(aq) + NH3(aq) + H2O(l)
• Washing soda • Manufacture of glass
• Softening of hard water • Manufacture of detergents
• Manufacture of soap • Manufacture of paper
• The solubility of KHCO3 is greater than that of NaHCO3 and cannot be precipitated by the above method. Therefore, K2CO3 could not be manufactured using Solvay process.
• In this process alternate layers of crushed CaCO3 and fuel (firewood) are fed into the kiln from top. A fire is started at the bottom and gradually spreads upward.
• The high temperature causes CO2 to be expelled from the kiln leaving CaO. After the kiln is cooled the quicklime is withdrawn from the bottom.
• Pollution due to the heat released to the environment.
• Air pollution caused by CO2 released and fine particles emitted.
CaCO3(s) ![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
Dissociation temperature of CaCO3 (900 °C) is relatively high, if the fuel (firewood) does not supply this temperature CaCO3 may not fully dissociate.
• If CO2 is not expelled fully from the kiln, it can combine with CaO again and form CaCO3 (as the reaction is reversible).
• CaO gets mixed with ash of the burnt fuel.
• Production of slaked lime and milk of lime
• Manufacture of calcium carbide
• Reducing acidity of the soil
• Manufacture of bleaching powder
• Construction of building
• Absorbing acidic gases
• Heating limestone to obtain quicklime (CaO)
• Sprinkling water on CaO to obtain slaked lime [Ca(OH)2(s)] and allow to cool
• Passing Cl2(g) over wet solid Ca(OH)2 at room temperature for about 12-15 hours in rotating kilns with intermittent raking
• Counter current principle is used for higher efficiency of the reaction.
CaCO3(s)![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
CaO(s) + H2O(l) → Ca(OH)2(s)
3Ca(OH)2(s) + 2Cl2(g) →Ca(OCl)2.Ca(OH)2.CaCl2.2H2O(s)
• Bleaching agent
• Disinfectant (especially for water)
• Quicklime (CaO) and coke (C) are heated in an electric arc at a temperature of
about 2000 °C.
CaO(s) + 3C(s) → CaC2(s) + CO(g)
2CaO(s) + 5C(s) → 2CaC2(s) + CO2(g)
• CaC2 reacts with H2O and produces C2H2.
CaC2(s) + H2O(l) → Ca(OH)2(s) + C2H2(g)
• In the production of oxyacetylene flame
• Used to induce flowering
• Used to induce ripening of fruits
• Nitrogen and hydrogen gases are used as raw materials.
• Nitrogen is obtained by the fractional distillation of liquid air.
• Hydrogen is obtained from naptha or natural gas as follows.
C6H14(g) + 6H2O(g) → 6CO(g) + 13H2(g)
in naptha
CH4(g) + H2O(g) → CO(g) + 3H2(g)
in natural gas
or partial oxidation with oxygen:
C6H14(g) + 3O2(g) → 6CO(g) + 7H2(g)
in naptha
2CH4(g) + O2(g) → 2CO(g) + 4H2(g)
in natural gas
• Nitrogen and hydrogen form an equilibrium mixture containing ammonia.
N2(g) + 3H2(g) ⇌ 2NH3(g) ΔH = -92 kJ mol-1
• Le Chatelier’s principle suggests that increase in pressure and decrease in temperature will increase the proportion of ammonia at equilibrium.
• High pressure obviously gives a high yield of ammonia, but the higher the pressure greater the cost and maintenance of equipment. The favoured pressure nowadays is 250 atm.
• The temperature must be low to give a higher yield of ammonia. But at low temperature the rate of reaction is so low that it makes the process uneconomical. In practice, the optimum temperature is usually about 450 0C. As the reaction is exothermic the system must be cooled.
• In addition to pressure and temperature, the catalyst is a vitally important variable. Here iron is used as a catalyst and small amounts of potassium oxide and aluminium oxide are used as promoters.
• Low concentrations of NH3 is favourable for the forward reaction. So NH3 is cooled under pressure and the liquid ammonia is removed.

• Production of nitric acid, fertilizers and nylon
• Petroleum industry utilizes ammonia in neutralizing the acid constituents of crude oil.
• Used in water and waste water treatment, such as pH control, in solution form to regenerate weak anion exchange resins.
• Used in stack emission control systems to neutralize sulphur oxides from combustion of sulphur-containing fuels.
• Used as a refrigerant in industrial refrigeration systems found in the food, beverage, petrochemical and cold storage industries.
• Used in the rubber industry for the stabilization of natural and synthetic latex to prevent premature coagulation.
• Ammonia and carbon dioxide are used as raw materials.
• Production of urea is a two step process.
(i) 2NH3(g) + CO2(g) ⇌ NH2COONH4(s)
(ii) NH2COONH4(s) ⇌ CO(NH2)2(aq) + H2O(l)
• Reaction of the step 1 is fast and exothermic and essentially goes to completion under reaction conditions used industrially. Unreacted NH3 and CO2 are fed into the first step.
• Reaction of step 2 is slower and endothermic and does not go to completion. The conversion is in the order of 50-80%.
• Urea is a popular solid nitrogen fertilizer because of its high nitrogen content (46%).
• Urea is used in the manufacture of urea-formaldehyde polymer.
• Ammonia, air and water are used as raw materials.
• The oxidation of ammonia by air to give nitric oxide is an exothermic reaction. The temperature is adjusted to and maintained at about 900 °C by controlling the flow rate of the gases.
• The process is operated under increased pressure because this packs more reactants into the same capacity plant and increases the rate slightly by increasing the number of molecular collisions per second at the catalyst surface.
• An excess air is used to ensure complete oxidation of NH3
• Cold air is added to the mixture as it leaves the catalyst because the next stage is an exothermic equilibrium and is therefore favoured by low temperature.
• Extensive cooling of the gases is necessary before nitrogen dioxide is absorbed in water in the presence of air to give nitric acid.
Thus the actual conditions used leading to about 96% conversion are
• Pressure: 1 – 9 atm
• Temperature : 850 -1225 0C
• Catalyst : platinum containing 10% rhodium
• Used in the synthesis of ammonium nitrate for use as a fertilizer and in explosives.
• Used to prepare nitrates which are of great importance in industry.
• NaNO3 is used as a preservative for meat.
• KNO3 is used in fertilizers and for making gun powder.
• AgNO3 is used to prepare photographic film and paper.
• For the preparation of aquaregia.
• Used to clean soldering surfaces
• Phosphorus is an essential nutrient for all living organisms.
• An important phosphorus containing fertilizer for plants is superphosphate which is a mixture of calcium dihydrogenphosphate Ca(H2PO4)2 and hydrated calcium sulphate (gypsum) CaSO4.2H2O.
• Eppawela apatite [3Ca3(PO4)2.CaX2 or Ca5(PO4)3X where X = F/Cl/OH] is a good raw material for the prodution of phosphate fertilzers.
• Apatite is insoluble and made soluble for short term crops by complete and partial acidulation.
• Sulphuric acid, nitric acid, hydrochloric acid and phosphoric acid can be used for acidulation.
3Ca3(PO4)2.CaX2(s) + 7H2SO4(aq) →3Ca(H2PO4sub>2)2(s) + 7CaSO4(s)+ 2HX(aq) —(1)
3Ca3(PO4)2.CaX2(s) + 14HCl(aq) → 3Ca(H2PO4)2(s) + 7CaCl2(s)+ 2HX(aq) —–(2)
• Apatite is finely ground and mixed with acid and left for 4-6 weeks. Then the product
single superphosphate (SSP) is obtained.
• Addition of ammonium sulphate to products of reaction (2) produces a non hygroscopic fertilizer.
CaCl2(s) + (NH4)2SO4(aq) → CaSO4(s) + 2NH4Cl(aq)
• Sulphur or sulphur containing minerals, air and water are used as raw materials. Sulphur dioxide produced during the extraction of metals such as lead and zinc from their sulphide ores can also be used.
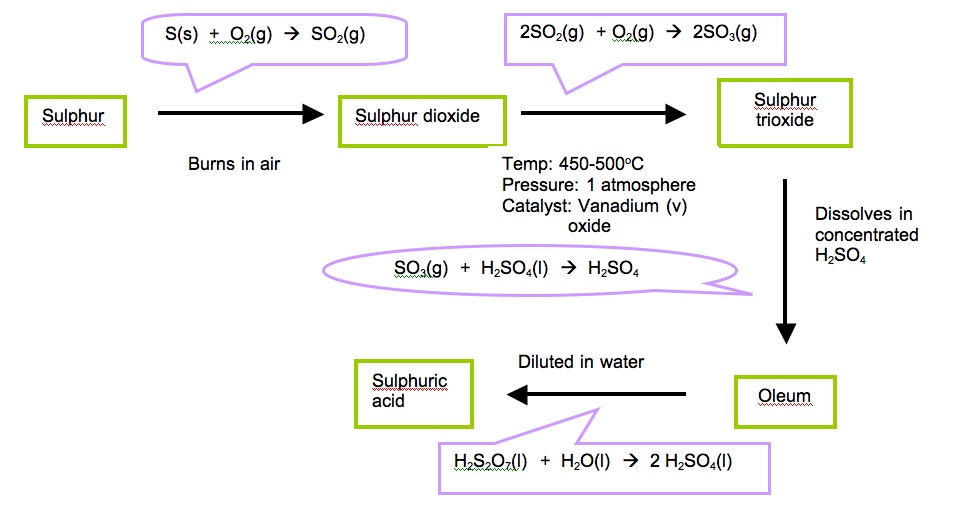
• The reaction between sulphur dioxide and oxygen is reversible. Sulphur trioxide continuously breaks down again to sulphur dioxide and oxygen. So the mixture is passed over several beds of catalyst to let the gases react again.
• The sulphur trioxide is removed between the last two beds of catalyst in order to increase the yield.
• As the reaction of formation of sulphur trioxide is exothermic and three moles of reactants form two moles of products, Le Chatelier’s principle predicts that the maximum yield of SO3 at equilibrium will be obtained at high pressure and at low temperature.
• In practice, a compromise temperature of 450 °C is chosen. This is the lowest that can be used without reducing the reaction rate to an unacceptable level. There are other reasons for keeping the temperature as low as possible. Fuel cost and corrosion of reaction chambers increase rapidly with rising temperature.
• At 450 °C conversion to SO3 is 97% and this high conversion even at atmospheric pressure, makes it unnecessary to carry out the process at increased pressure.
• As the reaction proceeds, the heat evolved in the exothermic reaction moves the systemto higher temperature. At this higher temperature the percentage conversion to SO3 ismuch reduced. Thus, it is necessary to cool gases between successive beds of catalyst.This is done using cold water pipes. The water is converted to steam and used to generate power(electricity).
• The sulphur trioxide is dissolved in concentrated acid rather than water. If it is dissolved in water, a thick mist of acid forms. This would be a pollution hazard.
• Oleum is mixed carefully with water to produce concentrated sulphuric acid.
• Manufacture of phosphate fertilizers
• Manufacture of ammonium sulphate fertilizers
• Manufacture of synthetic fibres rayon and plastics
• In the production of detergents – mostly alkyl and aryl sulphonates
• In the production of dyes, explosives and drugs
• In the production of battery acid
• Drying gases (Cl2)
• It is necessary to indicate concentration for solutions (e.g. Fe2+(aq, 1.0 mol dm--3) and pressure in case of gases e.g. H2(g, 1.0 atm).
When a piece of active metal such as magnesium is dipped in a beaker of water, there will be some tendency for the metal atoms to leave elctrons and go into solution as metal ions.The electrons will be left behind on the metal. In a very short time, there will be a build -up of electrons on the metal, and it will be surrounded in the solution by a layer of positive ions.These will tend to stay close because they are attracted to the negative charge on the piece of metal. This is called double layer as shown in figure (a).
• Some of them will be attracted enough so that they will reclaim their electrons and stick back on to the piece of metal. A dynamic equilibrium will be established when the rate at which ions are leaving the surface is exactly equal to the rate at which they are joining it again. Here, the double layer reach to its equilibrium. At this point there will be a constant negative charge on the metal and a constant number of metal ions present in the solution around it. However, at the dynamic equilibrium, ions are continually leaving and rejoining the surface with equal rates. The potential that is created between the metal and adjecent layers of solution is known as the electrode potential.
• If a less active metal such as copper is considered, it forms its ions less readily. Any ions which do break away are more likely to reclaim their electrons and stick back on to the metal again. It will still reach an equilibrium position, but there will be a lesser charge on the metal, and fewer ions in the solution.
If both equilibria are considered together it is clear that the position of the magnesium equilibrium Mg2+(aq) + 2e ⇌ Mg(s) lies further to the left than that of the copper equilibrium Cu2+(aq) + 2e ⇌ Cu(s)
• By IUPAC convention, all these equilibria are written with the electrons on the left hand side of the equation. It is recommennded to stick on to this convention.
eg Calomel electrode
Hg2Cl2(s) + 2e ⇌ 2Hg(s) + 2Cl–
eg Silver-Silver chloride electrode
 AgCl(s) + e ⇌ Ag(s) + Cl–
AgCl(s) + e ⇌ Ag(s) + Cl–
eg. Hydrogen electrode
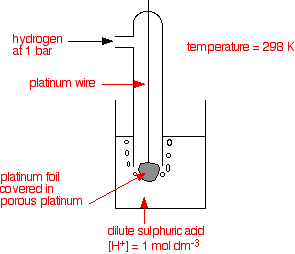 H+ + e ⇌ ½H2(g)
H+ + e ⇌ ½H2(g)
eg. Fe3+/Fe2+ electrode
Fe3+ + e ⇌ Fe2+
• The anode, or oxidation half-cell is always written on the left; the cathode, or reduction halfcell,is written on the right. The two electrodes are electrically connected by means of a salt bridge, denoted by two vertical bars.
• The cell terminals are at the extreme ends in the cell notation. Electrode and electrolyte interface of each half cell is separated by a single vertical bar. Other constituents are separated by commas.
Examples: Pt(s)|Fe2+(aq, 1 mol dm-3) , Fe3+(aq, 1 mol dm-3)
Hg(l),Hg2Cl2(s)|Cl(aq, 1 mol dm-3)
• When the half-reaction involves a gas, an inert material such as platinum serves as a terminal and as an electrode surface on which half-reaction occurs. The notation for the hydrogen electrode, written as a cathode and as an anode are given below respectively.
H+(aq, 1 mol dm-3)|H2(g)|Pt(s) || Pt(s)|H2(g)|H+(aq, 1 mol dm-3)
• Cell notations of several cells are given below.
Zn(s)|Zn2+(aq,1 mol dm-3 )||Cu2+(aq, 1 mol dm-3)|Cu(s)
Cd(s)|Cd2+(aq, 1 mol dm-3)||H+(aq, 1 mol dm-3)|H2(g)|Pt(s)
• The cell in which the cell reaction cannot be reversed by providing electrical energy is called a “primary cell’.
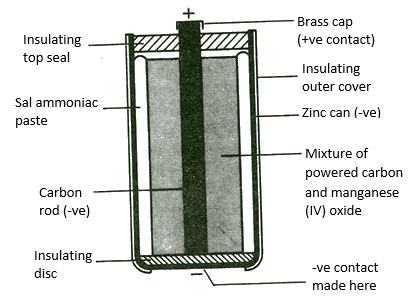
eg-Daniel cell
Electrolyte – NH4Cl/ZnCl2
(+) Pole – C/MnO2
(-) Pole – Zn
Reaction at (+) Pole(Cathode reaction) – 2NH4+(aq)+2MnO2(s)+2e → Mn2O3(s) + H2O(l)+2NH3(aq)
Reaction at (-) Pole(Anode reaction) – Zn(s) → Zn2+(aq) + 2e
Cell reaction – 2MnO2(s)+Zn(s)+H2O (l) → Zn2+(aq) + Mn2O3(s) +2OH–(aq)
Leclanche cell
Electrolyte -ZnSO4/CuSO4
(+) Pole – Cu
(-) Pole – Zn
Reaction at (+) Pole(Cathode reaction) – Cu2+(aq) + 2e → Cu(s)
Reaction at (-) Pole(Anode reaction) – Zn(s) → Zn2+(aq) + 2e
Cell reaction – Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
• A cell in which the reactants can be regenerated from the products by charging after
its discharge is referred to as a ‘secondary cell’.
eg. Lead accumulator

Electrolyte – dil. H2
SO4
Anode – Pb
Cathode – PbO2
Anode reaction during discharge – Pb(s) + SO42-(aq) → PbSO4(s) + 2e
Cathode reaction during discharge –
PbO2(s) + 4H+(aq) + SO42-(aq) + 2e → PbSO4(s) + 2H2O(l)
Electrochemcal cells in which reactants are continuously supplied from outside are referred as fuel cells. Dihydrogen and dioxygen, methane and dioxygen are commonly used for this purpose. One of the electrolyte used is concentrated KOH solution, maintained at 200 °C. Porous C-Ni electrodes are commonly used.
Relevent reactions for the dihydrogen-dioxygen fuel cell are given below.
Oxidation; 2H2(g) + 4OH–(aq) → 4H2O(l) + 4e
Reduction; O2(g) + 2H2O(aq) + 4e → 4OH–(aq)
Cell reaction 2H2(g) + O2(g) → 2H2O(l)
• Electrolysis is the passage of a direct current through a substance that is either molten or dissolved in a suitable solvent, resulting in chemical reactions at the electrodes and separation of mixtures.

• An ion-electron half reaction occurs at each electrode.
• The overall reaction is a redox reaction.
• The terminal at which oxidation occurs is the anode whereas the terminal at which reduction occurs is the cathode .
• The electrode connected to the positive terminal of the external source of electricity is the positive electrode. The electrode connected to the negative terminal is the negative electrode.
• The positive ions in the solution are attracted by the negative electrode. The negative ions are attracted by the positive electrode.
• Under certain conditions, a different species in the medium/set up may be oxidized or reduced in preference to the relevant ions.
Electrolysis of water (acidic or basic) using inert (carbon or platinum) electrodes
At anode; 2H2O(l) → O2(g) + 4H+(aq) + 4e
At cathode; 2H2O(l) + 2e → H2(g) + 2OH–(aq)
Electrolysis of aqueous CuSO4 solution using copper electrodes
At anode; Cu(s) → Cu2+(aq) + 2e
At cathode; Cu2+(aq) + 2e → Cu(s)
Electrolysis of aqueous CuSO4 solution using inert electrodes
At anode; 2H2O(l) → O2(g) + 4H+(aq) + 4e
At cathode; Cu2+(aq) + 2e → Cu(s)
Electrolysis of aqueous NaCl solution using inert electrodes
At anode; 2Cl–(aq) → Cl2(g) + 2e
At cathode; 2H2O(l) + 2e → H2(g) + 2OH–(aq)
Electrolysis of molten NaCl using inert electrodes
At anode; 2Cl–(l) → Cl2(g) + 2e
At cathode; Na+(l) + e → Na(l)
(1) The quantity of a substance liberated at an electrode is directly proportional to the quantity of electric charge that has flowed in the circuit.
(2) For a given quantity of electric charge, the amount of any metal deposited is proportional to its equivalent weight (atomic weight devided by the charge on the metal ion)
Faraday’s constant (F) = Molar charge of a proton = 1.602 x 10-19 C x 6.022 x 1023 mol-1= 96 484 C mol-1≅96 500 C mol-1
• Electroplating means coating a metal with another metal using electricity. This is different from the deposition of a less reactive metal on a more reactive metal.
In order to have a quality coating during electrolysis, the coating should strongly bind with the metal. In addition, it should have the following qualities.
• Strength
• Lustre
• Chemical inertness
• Good mechanical properties
• Absence of cracks and holes
• Uniformity in thickness and appearance
To get a quality metal coating the following factors should be appropriately controlled.
• The nature and purity of the electrolyte
• Concentration of ions
• Temperature
• Current density
• pH value
• Nature of the other ions presents
• Potential difference
• Purity of the anode
• Relative positioning of the anode and the cathode
• Cleanliness of the object and the nature of its surface
Two or more reactions may also occur simultaneously on the electrodes. They can be controlled by changing the temperature, concentration, voltage and the nature of electrodes.
In bimetallic corrosion, the more reactive metal corrodes faster in case where a pair of metals with electrical and physical contact is dipped in a corrosive electrolyte. The less corrosion resistant or the “active” member of the couple experiences accelerated corrosion while the more corrosion resistant or the “noble” member of the couple experiences reduced corrosion due to the “cathodic protection” effect. The most severe attack occurs at the joint between the two dissimilar metals. Further away from the bi metallic joint, the degree of accelerated attack is reduced.
It is a technique to control the corrosion of a metal surface by making it work as a cathode of an electrochemical cell. This is achieved by placing in contact with the metal to be protected another more easily corroded metal to act as the anode of the electrochemical cell.
Cathodic protection systems are most commonly used to protect steel, water or fuel pipelines and storage tanks, steel pier piles, ships, offshore oil platforms and onshore oil well casings.
Galvanizing of iron is an another example.
Protection of a metal by rendering its surface inactive by chemical methods is called passivation.
In the context of corrosion, passivation is the spontaneous formation of a hard non reactive surface film that inhibits further corrosion. This layer is usually an oxide or nitride that is a few molecules thick.
Stainless Steels can be passivated using a solution of nitric acid, to remove foreign particles from the surface and promote the growth of a protective oxide layer. Nickel can be used for handling elemental fluorine, due to a passivation layer of nickel fluoride.
• Strong electrolytes
Strong electrolytes are substances that are virtually fully ionized in solution, and include ionic solids and strong acids. As a result of their complete ionization, the concentration of ions in solution is proportional to the concentration of strong electrolyte added.
eg. NaCl, KNO3, HCl in aqueous solutions
• Weak electrolytes
Weak electrolytes are not fully ionized in solution. They include weak Bronsted acids and bases.
eg. CH3COOH, NH3, H2O
• Non-electrolytes
Liquids/solutions that do not contain ions are referred as non electrolytes. They do not conduct electricity.
eg. C6H6, Kerosene
A metal cuboid is shown on the left hand side above. A cuboid shape portion of a solution with the similar dimensions of the metal cube which is in between two electrodes is shown on the right hand side.
Here l = Length of the metal cube or the selected cuboid shape portion of the solution (m)
A = Area of cross section (m2)
R = Resistance (Ω )
ρ= Resistivity (Ωm )
1/R = Conductance ( Ω-1 or S )
Regarding the above cuboids,
R∝l and R∝ (1/A)
∴ R∝(l/A)
R = ρl/A
ρ = RA/l
k =1/ρ = l/AR
• Coherent SI unit of conductivity is and the most practical unit is Ω-1m-1
• Conductivity and resistivity are constants for the particular substance (metal or solution with a given concentration) and it changes with temperature (about 2% per one degree Celsius in solution).
• Nature of the solute (aqueous solutions of strong, weak and non electrolytes, molten electrolytes)
• Concentration of the solute
• Temperature
• For dilute solutions decrease in conductivity is approximately proportional to the concentration and this is very correct for very dilute solutions. The reason is the decrease of interactions among ions in dilution.
• Current = Charge / Time.
Current carried by an ion at a given temperature in a given electric field depends on the concentration of ions and their speed. Speed of an ion depends on its charge, size and potential gradient of the applied electric field.
• H+ and OH– ions have the highest speeds. So those ions contributes a lot for the conductance. For example, H+ ions contribute about 80% for the conductance of dilute HCl solution.
• The potential difference in the double layer of a given electrode is considered its electrode potential. This absolute difference cannot be measured. It can only be measured relative to another electrode.
• An electrode with a known or a defined potential used to measure the potential of a given electrode is called a reference electrode. Standard electrode potentials are measured with reference to the standard hydrogen electrode. The potential of the standard hydrogen electrode is defined as 0.00 V {[H+ (aq)] is 1.0 mol dm-3 and pressure of H2(g) is 1 atm and temperature is 298 K}. Calomel electrode and AgCl(s)|Ag(s) electrode are used as practical reference cells. Standard electrode potentials of Calomel electrode and AgCl(s)|Ag(s) electrode are +0.22 V and +0.2415V respectively.
• The value of the potential of a given electrode under standard conditions measured relative to the standard hydrogen electrode (SHE) is its standard electrode potential.
• The following diagram illustrates a set up that can be used for measuring the standard electrode potential of the Zn2+ (aq)|Zn(s) electrode.

• When the standard electrode potentials (reduction potential) of different electrodes are arranged in the ascending order, the electrochemical series is obtained.
Factors affecting the electrode potential
• Temperature
• Concentration of the electrolyte
• Nature of electrolyte
• Pressure (of a gas)
• Type of electrode
• Electromotive force (e.m.f.) is defined as the potential difference between the two electrodes when no current flows through the cell.
• Temperature, concentration of the electrolyte, electrode type and the nature of the electrolyte are the factors that affect the electromotive force. It is independent of the distance betweenthe electrodes and the surface area of the electrodes.
• Electrolytes in the two electrodes of an electrochemical cell are connected to complete the internal circuit through a salt bridge or a permeable membrane/diaphragm/porous partition.A tube filled with a solution of a salt such as KCl or NH4NO3 which is gelified with agar is used as a salt bridge. Such an electrochemical cell is known as a cell with a liquid junction.
• In a cell, when both electrodes are not separated by a salt bridge or a permeable membrane/diaphragm/porous partition is referred as a cell without a liquid junction.
• According to the international convention, electrode potentials are measured relative to the standard hydrogen electrode. On this standard, the electrode potential of the standard silver-silver chloride electrode is 0.22 V. Similarly, the electrode potentials of other standard electrodes can also be calculated. Show that in arranging these in the ascending order, electrochemical series is obtained.
Ecell = Ecathode – Eanode