Length – Meter ruler , Vernier caliper , Micrometer screw guage , Spectrometer , Travelling microscope
Mass – Electronic balance , Triple beam balance
Time – Stop watch , Digital clock
Least count
Vernier caliper
Internal jaws – for measuring inner dimensions
External jaws – for measuring outer dimensions
Depth bar – for measuring depths
Reading =main scale reading + vernier scale coincide × least count = 100+2×0.1=100.2 mm
Zero error and correction
The instrument is said to have if the zero of the main scale doesn’t coincide with the zero of the vernier scale when the two jaws of vernier caliper are brought into contact.
Zero error = 0.3 mm
Correction = -0.3 mm
Zero error = 0.8 mm
Correction = +0.8 mm
Pitch – It is the linear distance moved by thimble along the main scale when the thimble is given one rotation.
Least count = Pitch/Number of divisions in the circular scale
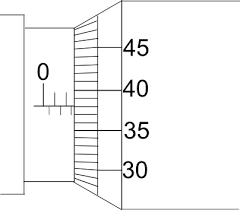 Main scale – 0.5 mm divisions Least count=0.01mm
Main scale – 0.5 mm divisions Least count=0.01mm
Reading = 2.5 + 38 ×0.01 = 2.88 mm
Zero error and correction
Spherometer
Pitch = It’s the linear distance moved along main scale when circular scale is given one completed rotation.
Least count = Pitch/Number of divisions in the circular scale.thDetermination of the radius of curvature of a spherical surface
R = a2/6h + h/2
a = distance between two legs
Travelling microscope
The principle component of a travelling microscope is it’s microscope. It enlarges the diameter so measurement can be done easily.

There are three types of physical quantities.
The internationally accepted standard units and dimensions of the physical quantities are given below.
Fundamental physical quantities SI unit Dimensions
Supplementary physical quantities
Derived physical quantities
| Physical quantity | Unit | Dimesion |
|---|---|---|
| Speed | ms-1 | LT-1 |
| Velocity | ms-1 | LT-1 |
| Acceleration | ms-2 | LT-2 |
| Pressure | kgm-1s-2 =Pa | ML-1T-2 |
| Work | kgm2s-2=J | ML2T-2 |
| Energy | kgm2s-2=J | ML2T-2 |
| Power | kgm2s-3=W | ML2T-3 |
| Frequency | s-1=Hz | T-1 |
| Area | m2 | L2 |
| Volume | m3 | L3 |
| Density | kgm-3 | ML-3 |
Pysical quantities which can be defined in terms of fundamental quantities are called derived quantities.
Scalar quantities
Physical quantities which has magnitude are called scalars.
Vector quantities
Physical quantities which have magnitude and direction are called vectors.
Any vector can be represented by a segment of straight line.Length of the straight line represents the magnitude of the vector and direction of the straight line represents the direction of vector.
Vector addition
Gaseous composition of dry air.
| Constituent | Chemical symbol | % by volume |
|---|---|---|
| Nitrogen | N2 | 78.084 |
| Oxygen | O2 | 20.947 |
| Argon | Ar | 0.934 |
| Carbon dioxide | CO2 | 0.0350 |
• Also present are smaller amounts of Ne, He, CH4, Kr, H2 and in addition variable trace quantities of other pollutants such as NH3, SO2, CO, NO2, O3 and H2S. Water vapour present to about 4% in the atmosphere may not be uniformly distributed (may vary from time to time) but concentrated close to the oceans and large water bodies.
• The amount of N2 in the atmosphere is high because of its inertness due to the presence of strong N ≡N bond. Reactivity of O2 is greater than N2 and the amount of O2 is smaller than N2i n the atmosphere. Presence of O2 makes the atmosphere to be active and sustains life on the earth.
• CO2 and H2O are the main ingredients for photosynthesis.
Variation of atmospheric temperature, molar mass and pressure according to the altitude
• With the altitude mass is gradually decreasing and accordingly pressure is also coming down.But the temperature undergoes several inversions. Based on these thermal inversions atmosphere is divided into different regions. The region closer to the ground is the troposphere and the region above the troposphere is stratosphere. Ozone layer is located in the stratosphere.
• Water covers 70% of the earth’s surface. Very little of the world’s water is fresh water (2.6%).Most of the water (97.4%) is in the oceans. Most of the fresh water (76%) is frozen inglaciers and in the polar ice caps. Only a tiny fraction (0.01%) is available for human use.
Major cycles
• Knowledge of chemical cycles is very important to understand the fate of the chemicals, their abundance in different spheres, their possible environmental impacts and controlling the pollution problems.
Carbon cycle
• The only way that carbon gets into ecosystem is through photosynthesis.
• Animal gain carbon through their food.
• Decomposers get their carbon by digesting dead organisms.
• All living organisms return carbon to the air in the form of carbon dioxide through
respiration.
• If plants or animals die in situations where there are no decomposers (E.g. : deep
oceans) the carbon in them can get turned into fossil fuels over millions of years.
• The carbon in fossil fuels is released during the combustion.
• Microorganisms are important in the carbon cycle because they can quickly get the
carbon in dead material back into the atmosphere
Oxygen cycle

• Atmospheric oxygen is removed through combustion (chemical / biological) and respiration and replenished through photosynthesis.
• Most oxygen is stored in the oxide minerals of the earth crust and mantle but is bound to rocks and unavailable for use.
• Most available oxygen comes from photosynthesis and some is made in the atmosphere when sunlight breaks down (photolysis) water.
Nitrogen cycle
• Atmospheric nitrogen is fixed by bacteria. Some live free in the soil (Eg:- Azotobacter).Others like Rhizobium are found inside root nodules of leguminous plants (hat’s peas,beans and cloves). Atmospheric nitrogen is changed into ammonia, nitrites and then nitrates which all plants can absorb and use to make protein.
• Nitrogen in the plant proteins is passed onto animals through food chains.
• When living organisms die their nitrogen is returned to the soil in the form of ammonium compounds by micro organisms. Animals get rid of excess aminoacids via deamination in their liver. The nitrogen gets back into the soil via their urine.
• Ammonium compounds are changed into nitrates by nitrifying bacteria. Firstly nitrosomonas changes ammonium compounds into nitrites, then Nitrobacter changes the compounds into nitrates.
• Nitrates are converted back into atmospheric nitrogen by denitrifying bacteria like
Pseudomonas and Thiobacillus.
Hydrological cycle
• Optimum compositions of atmosphere, hydrosphere and earth’s surface are important on environmental equilibrium for sustainability of earth. If this equilibrium is disturbed the following problems could occur.
• Damaging effects on human health
• Damage on plants and hindrance of their growth
• Damage on marble buildings and metallic structures
• Increase in salinity / alkalinity
• Weathering of rocks
• Climatic changes (may cause drought / flood)
• CO(g), H2S(g), SO2(g), SO3(g), NO(g), NO2(g) and CO2(g) are the inorganic compounds which change the atmospheric composition. Organic compounds including hydrocarbons, halo hydrocarbons and particles such as dust and carbon also contribute to change the composition of the atmosphere.
• Some CO(g) in atmosphere is formed by the oxidation of methane, which is formed naturally by the anaerobic degradation of organic matter. CO (g) is emitted from all the incomplete combustion processes including the internal combustion engines of motor vehicles.
• SO2(g) enters the atmosphere from the combustion of sulphur containing fossil fuels,volcanic eruption , biological decay of S containing organic matter, reduction of sulphates and recovery of metals from their sulphides. SO2(g) reacts with oxygen and forms SO3(g). NO(g) increases the rate of oxidation of atmospheric SO2 (g) in to SO3(g).
• NOx(g) [NO(g) and NO2(g)] enters the atmosphere from natural processes such as lightening discharges, and from pollutant sources. The combustion of fossil fuels gives most of the NOx(g). Much of the NOx(g) entering the atmosphere is from the internal combustion engines.
• Microbial decay of S containing organic matter and reduction of sulphate ion are the most common natural sources of H2S.
• Hydrocarbons are widely used as fuels and enter the atmosphere directly or as byproducts in the partial combustion of other hydrocarbons. Uncontrolled vehicle exhausts contain alkanes , alkenes and aromatic hydrocarbons. Methane is produced in large quantities from the anaerobic decomposition of organic matter submerged in water.
Greenhouse effect
• The temperature of the earth is fixed by a steady – state balance between the energy received from the sun and the energy radiated back by the earth. One mechanism for regulating the earth’s temperature is the greenhouse effect.
• The loss of energy from the earth is achieved by means of conduction, convection and radiation. A fraction of the earth’s heat is transmitted to clouds by conduction and convection before being lost by radiation.
• Convection carries heat in the form of the enthalpy of vaporization of water. The water vapour releases heat as it condenses.
• The radiation that carries energy away from the earth is of longer wavelength, in the infrared region.
• If all the outgoing radiation were able to escape, the surface of the earth would be at -16 °C (Same temperature as in the moon).
• Most heteroatomic molecules and some homoatomic molecules (O3) are known as greenhouse gases and these are the ones which contribute to the greenhouse effect.
• Accordingly CO2(g), water vapour, methane, dinitrogen oxide, ozone, SO2 and CFCs absorb radiation given out from the earth and some of it re-radiate back to the earth’s surface. This re-radiation helps to warm the earth andmaintain a climate that will support life. This is called greenhouse effect and these gases are called greenhouse gases.
1. Global warming
• When the greenhouse gases exceed their permissible level. Hence, the temperature of the atmosphere increases. This is called “Global Warming”.
• CO2(g) plays the key role in this global warming. Gases such as NOx and CFCs are the other examples. Though the CFC levels are low they have a longer residence time and they have a high efficiency of absorbing IR radiations. Therefore, their contribution is high.
• The results of global warming include melting of polar ice caps, sinking of low lying countries due to the thermal expansion of sea water, desertification due to loss of soil moisture, drying of fresh water reserviors, changes in biodiversity and weather patterns.
• A significant amount of atmospheric carbon dioxide dissolves in water. Thus, it reduces the contribution of CO2 to global warming. However, with the increase of temperature dissolution of carbon dioxide is reduced and dissolved carbon dioxide returns to the atmosphere.
• Increasing CO2 level in the atmosphere increases the photosynthesis. This is a positive effect of global warming.
• As far as Sri Lanka is concerned global warming can have a higher effect because we are an island situated closer to the equator.
2. Αcid rain
Acidic gases in the atmosphere dissolves in water to contribute to the acidity. Two important factors are
(i) Dissolution of acidic gases in water
(ii) Strength of the resulting acid
In this context even though CO2 levels are high, their contribution to the acidity is very low (pH 5.1 – 5.8) and it is not considered as acid rain. But the SOx and NOx even though they are present in smaller quantities have a higher contribution resulting in a pH of 4 – 5.
Reactions of atmospheric SO2 (g)
(i) SO2(g) + H2O(l) ![]() H2SO3(aq)
H2SO3(aq)
H2SO3(aq) + H2O(l) ![]() H3O+(aq) + HSO3–(aq)
H3O+(aq) + HSO3–(aq)
HSO3–(aq) + H2O(l) ![]() H3O+(aq) + SO32-(aq)
H3O+(aq) + SO32-(aq)
(ii) Oxidants in the atmosphere can oxidize SO2 to SO3.
SO2(g) → SO3(g)
O2(g), O(g), OH(g) and peroxides can serve as oxidants. Some salts can catalyse the oxidation. Then SO3(g) dissolves in H2O to form H2SO4.
(iii) Both SO2 and the oxidant (normally O2) can dissolve in a rain drop. Rain drop facilitates the oxidation process by bringing two chemicals together.
2SO2(aq) + 2H2O(l) + O2(aq) → 2H2SO4(aq)
H2SO4(aq) + 2H2O(l) → 2H3O+(aq) + SO42-(aq)
• Similarly NOx reacts in the atmosphere.
2NO(g) + O2(g) → 2NO2(g) 4NO2(aq) + 2H2O(l) + O2(aq) → 4HNO3(aq)
HNO3(aq) → H+(aq) + NO3–(aq)
• Acid rain damages plants and causes the death of fish in the lakes. Acids such as sulphuric acid and nitric acid dissolve aluminium from aluminosilicate materials of soil giving free Al3+ to water. It interferes with the operation of fish gills.
• Acid rain water, draining through soils washes out nutrients and liberates aluminum ions.The roots of the trees may take up the aluminum ions instead of essential nutrients.
eg. Ca2+ and Mg2+.
• Limestone, metallic structures, bridges, ships, motor vehicles, etc. are also affected.
Change of the composition of earth’s surface due to acid rain
• Dolomite, limestone or marble are soluble in acidic water.
• Under mild acidic conditions;
CaCO3(s) + H+(aq) → Ca2+ (aq) + HCO3–(aq)
MgCO3(s) + H+(aq) → Mg2+(aq) + HCO3–(aq)
CaCO3.MgCO3(s) + 2H+(aq) → Ca2+(aq) + Mg2+ (aq) + 2HCO3–(aq)
Here, insoluble substances become soluble.
• Under strong acidic conditions;
CaCO3(s) + 2H+(aq) → Ca2+(aq) + H2O(l) + CO2(g)
MgCO3(s) + 2H+(aq) → Mg2+(aq) + H2O(l) + CO2(g)
CaCO3.MgCO3(s) + 4H+(aq) → Ca2+(aq) + Mg2+(aq) + 2H2O(l) + 2CO2(g)
• Many other salts in the rocks and sand also dissolve in the acid rain. Soil becomes gradually more acidic in the natural course of events. Cations are removed from the soil solution by plants and replaced by H+ ions. Minerals such as sulphides are oxidized to form acids. At low pH, hydrogen ions displace other cations from soil. Not only Al3+, Mg2+, Ca2+ but heavy metal ions are also displaced by H+ ions. The leaving of these ions deprives plants of the nutrients required for healthy growth. The acidic water passing through the soil, causes to leaching Al3+ and other minerals and also weathering of rocks. The Ca2+ and Mg2+ concentration increase in water, and hardness of water also increases. The acidity, salinity and nitrogen concentration also increase insurface water. Concentration of the heavy metal ions also increases in the surface water.
3. Photochemical smog
• Motor vehicle emissions contain NOx and unburnt hydrocarbons (CxHy). They are converted to ozone, aldehydes, peroxyacetyl nitrate (PAN), peroxy benzoyl nitrate (PBN), etc. in the presence of sunlight and temperatures above 15 °C.
• This is known as photochemical smog as those chemicals are formed in the presence of sunlight
• Smog is a yellowish haze which reduces visibility and causes eye irritation.
• The word smog is used to describe the combination of smoke and fog.
• The starting reaction of photochemical smog is dissociation of NO2 to NO and ‘O’.
• The steps in the formation of a photochemical smog are given below.
(i) NO2 absorbs sunlight and undergoes photolysis.
NO2 hυ→ NO + O
(ii) The resulting atomic oxygen combines with O2 molecules
a) to form ozone.
O + O2 + M → O3 + M
(M is known as third body which absorbs excess energy. M can be an airborne particle
or a gas.)
b) to form OH radicals.
O + H2O → 2OH•
(iii) The resulting HO• converts other airborne chemicals into radicals and they start a set of reactions to produce aldehydes, PAN, PBN, etc.
Effects of photochemical smog are given below.
• Effects on human health and comfort : Photochemical smog affects the respiratory system.It causes coughing, wheezing, etc.
• Damage to materials : Ozone causes rubber to deteriorate through fission of the double bond and also reduces the quality of fabrics and bleaches dyes.
• Effects on the atmosphere : Aerosol particles scatter light and reduce the visibility.
• Toxicity to plants : Most of the photochemical smog products are toxic to plants. Plant growth is inhibited by the prodcts from photochemical smog. This can effect the food production.
4. Depletion of ozone layer
• There is a layer of ozone in the stratosphere. The ozone layer prevents high energy UV light from reaching the troposphere.
• Some reactions involving O2(g) and O3(g) are;
a) The O2(g) is dissociated by solar UV radiations.
b) Some of the atomic oxygen (O) combines with dioxygen molecules to form trioxygen molecules (O3).
O (g) + O2(g) + M → O3(g) + M
c) O3(g) absorbs UV light with different frequencies and dissociates.
O3(g) → O2(g) + O(g)
d) O3 molecule reacts with O atom and forms O2 molecule.
O3(g) + O(g) → 2O2(g)
There is a natural balance which keeps the ozone layer at a constant thickness.
• Ozone is destroyed by reactions with and other free radicals. These radicals act as catalysts and destroy thousands of O3 molecules. The catalysed destruction of ozone in the stratosphere is known as ozone layer depletion.
• Chlorine radicals from the chlorofluorocarbon have been recognized as a major contribution to ozone layer depletion . This chloroflurocarbons are stable in the atmosphere but produce radicals in stratosphere with UV radiation.
• There is a strong connection between UV radiation and the cataract formation as well as incidence of both non-fatal and fatal skin cancer in humans. The ozone layer protects us.
To minimize the environmental and health effect of the above mentioned global issues, emission of pollutant gases has to be minimized. Such several remedial actions are given below.
• Minimization of fuel combustion
Motor vehicles, industries and routine household activities (such as cooking) releases large amount of carbon dioxide to the environment. Some of the releases can be controlled. For example, we can reduce drastically the number of vehicles on our roads. The development of an efficient public transport system involving electric trains and electric cars is an alternative.Using fuels of lower carbon to hydrogen ratio will minimize the CO2 in combustion. Use of the other energy sources such as nuclear and solar energy instead of fossil fuel is another option. Proper vehicle inspection and burning fuel only (without impurities) when necessary will help in this issue.
• Absorption of CO2 by trees
Carbon dioxide produced by the respiration of living organisms and the normal activities of man is fixed by the green plants during the photosynthesis. Photosynthetic organisms utilize solar radiation to convert carbon dioxide and water to carbohydrates using a chlorophyll catalyst.
6 CO2(g) + 6 H2O(l) → C6H12O6(s) + 6 O2(g)
• Here green plants in a way purify our air since oxygen is produced as a byproduct of
photosynthesis.
• Tropical rain forests are warm and humid. These conditions are ideal for photosynthesis.Their destruction is one of the factors that is causing an increase in atmospheric carbon dioxide level. Therefore, preservation of forest areas and planting are the best ways of controlling CO2 increase.
• Complete combustion
• Carbon monoxide is a major air pollutant which is formed due to incomplete combustion of fuels. The largest amount of CO comes from motor vehicle exhaust.
• The combustion of butane, for example requires 6.5 moles of oxygen per mole of hydrocarbon. If only six moles of oxygen are present one mole of CO will result.
2C4H10(g) + 13 O2(g) → 8 CO2(g) + 10 H2O(g)
C4H10 (g)+ 6 O2(g) → 3 CO2(g) + CO(g) + 5 H2O(g)
• Maintaining the air / fuel ratio(by mass) leads to a complete combustion.The equation for the complete combustion of octane is;
2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(g)
From the stoichiometry of the equation it follows that;
(mass of air)/(mass of octane) = 14 : 7. This is called the air/fuel ratio
.
• A rich mixture [which is having more hydrocarbons (fuels) or having oxygen less thanthe stoichiometric proportion] gives an exhaust gas which is high in CO and partially combusted organic products. A lean mixture (with an excess of air or less fuel) gives an exhaust gas with less CO but has more oxides of nitrogen (NOx). The best way to control the emissions is “tuning up” (adjusting the air to fuel ratio for the optimum condition) and the use of a catalytic converter to convert the pollutants to harmless products.
• The control of emission from the internal combustion engine is the best hope of reducing CO level.
• Several soil microorganisms have enzymes which catalyze the oxidation and remove CO from the atmosphere.
• Both N and S will form different oxides and they are acidic in nature. Hence the burning of any material containing N or S in air can produce SO2 and NOx. Atmospheric N2 is not reactive due to a strong triple bond between two nitrogen
atoms. But, if the temperature is greater than 900 °C this bond can be cleaved forming NOx’s (NO and NO2). The burning temperature exceeds 900 °C in most of the combustions including internal combustion engines, burning cigarettes and in the cooking stoves. Also it happens naturally, with thundering and lightening. The best way to minimize the emissions of SO2 and NOx is lowering the temperature of the combustion process and reducing the burning of S and N containing material.
Following methods can also be used to reduce the release of acidic gases to the atmosphere.
• Absorption methods
Acidic gases can be neutralized by reacting with a base. We have enough natural bases such as limestone (CaCO3) and magnesium oxide (MgO) that can be used to remove (scrub) the acidic gases. The products that are formed can be converted to the valuable industrial chemical, sulphuric acid.
(i) Slurry of limestone and lime is used to “scrub” the fuel gases.
CaCO3(s) + SO2(g) → CaSO3(s) + CO2(g)
CaO(s) + SO2(g) → CaSO3(s)
2 CaSO3(s) + O2(g) + 2 H 2O(l) → 2 CaSO4.2H 2O(s)
(ii) Slurry of magnesium oxide is used as a scrubber.
MgO(s) + SO2(g) → MgSO3(s) →Δ MgO(s) + SO2(g)
The MgSO3 is heated to give MgO which is recycled and SO2 at a concentration high enough is used in the manufacture of sulphuric acid.
(iii) A solution of sodium sulphite can be used for scrubbing.
Na2SO3(s) + H2O(l) + SO2(g) → 2NaHSO3(aq)
The NaHSO3 produced can be heated to give Na2SO3 for recycling and SO2 can be sold to sulphuric acid manufacturers.
• Minimization of pollutant gases from car exhaust
The most significant pollutant gases in vehicle exhausts are CO, NOx and un-burnt or partially burned hydrocarbons. The partial combustion is due to lack of oxygen. This can be reduced by adjusting the air to fuel ratio as discussed above and this is known as “tuning up” of a vehicle. Toxic gases in automobile exhaust fumes could be controlled by installing catalytic converters along the exhaust pipes of the vehicles. An efficient catalytic converter should oxidize carbon monoxide and unburnt hydrocarbons to carbon dioxide and water and also reduce nitric oxide and nitrogen dioxide to nitrogen and oxygen. These oxidations and reductions are done at two stages on the catalytic surfaces of the catalytic converter which is fixed to the silencer (muffler) of a vehicle. Hot exhaust gases are fed through catalytic converter containing thin film of an inert metal such as platinum and transition metal oxides such as copper and chromium oxides.
2 NO(g) + 2 CO(g) → N2 (g) + 2 CO2(g)
2 CO(g) + O2(g) → 2 CO2(g)
C7H16(g) + 11 O2(g) → 7 CO2(g) + 8 H2O(l)
Three way catalytic converters (oxygen monitor fitted) transform harmful exhausts of CO,NOx and CxHy to relatively harmless N2, CO2 and H2O. The catalytic converters do not start working until the catalyst has reached a temperature about 200 °C. So they are not effective until the engine has warmed up.
Water quality
Physical Parameters
(i) Temperature
Temperature should less than 40 0C. Hot water accelerates the biological processes. It reduces the dissolved O2 and affects the aquatic organisms.
(ii) pH Value
• A pH range of 6.0 – 9.0 appears to provide protection for the life of fresh water fish and bottom dwelling invertebrates.
• pH of water may also be changed due to dissolved gas from air such as CO2, SO2 and the mixing with industrial effluents.
• Normal pH range of ground water which is useful is 6.0 – 8.5.
If pH < 6.5 it is acidic water which is corrosive. Usually soda ash is used to neutralise the acidity.
• Dolomite can also be used in agriculture. Dolomite slowly neutralises acidity of water.
2H+(aq) + CaCO3.MgCO3(s) → Ca2+(aq) + Mg2+(aq) + 2 HCO3–(aq)
(iii) Conductivity
• Conductivity is a measure of the ability of an aqueous solution to conduct an electric current.
• Conductivity depends on the following factors.
• Concentration of ions • Mobility of ions
• Oxidation state • Temperature of water
• The unit used to measure the conductance is ohm-1 = Ω-1 or Siemens (S). Conductance = 1/Resistance
This measures the ionic strength but does not identify the ions present.
(iv) Turbidity
Sediments in the water prevents the penetration of light to the bottom of water bodies. This will reduce the photosynthesis activity and also creates anaerobic environment introducing a bad smell to the water.
(v) Dissolved amount of oxygen and water quality
• O2 is required for the metabolism of aerobic organisms and also influences some chemical reactions.
• O2 in air dissolves in water. Photosynthesis is the process of producing O2.
• Concentration of dissolved O2 decreases as temperature increases.
• Submerged green plants and algae increase the dissolved O2 content in a water body in the day time.
• Decaying organic matter consumes dissolved O2 in a water body.
Chemical oxygen demand (COD)
(CHO)n(aq) + O2(g) → x CO2(g) + y H2O(l)
• Amount of oxygen required in mg dm-3 for this oxidation of organic matter wastes to be carried out chemically is called chemical oxygen demand (COD).
• Since the amount of O2 consumed cannot be easily quantified, dichromate is used for laboratory determination. In this determination the sample is heated with an acid solution of potassium dichromate. All oxidizable organic matter will react with dichromate at this stage.Silver sulphate is generally added as a catalyst.
• Chloride present in water can be oxidized to chlorine under these conditions by dichromate.This is prevented by converting chloride to undissociated mercuric chloride. The excess of unreacted dichromate is determined by titrating with iron(II) ammonium sulphate.
Cr2O72-(aq) + 14H+(aq) + 6e → 2Cr3+(aq) + 7H2O(l)
• Ιn acidic medium;
O2 (g) + 4H+(aq) + 4e → 2H2O(l) (CHO)n(aq) + O2(g) → x CO2(g) + y H2O(l)
• If the organic matter is oxidized by acid dichromate, then
Cr2O72-(aq) + 14H+(aq) + 6e → 2Cr3+(aq) + 7H2O(l)
1 mol O2(aq) ≡ 2/3 mol of Cr2O72-(aq)
Biochemical oxygen demand / Biological oxygen demand (BOD)
(CHO)n(aq) + O2(g) → x CO2(g) + y H2O(l)
• Amount of O2 required to be carried out for the above oxidation by microorganisms is called biochemical oxygen demand.
• The consumed dissolved oxygen can be used to determine the BOD value.
• Substances which use up dissolved oxygen will contribute to the value of BOD. Pollutants such as human and animal wastes, food canneries, meat etc. controls the BOD.
• When a water sample is saturated with oxygen, the initial concentration of dissolved oxygen can be determined. If it is incubated at 20 0C for a known period, usually 5 days, the microorganisms in the water oxidize the organic matter. The oxygen that remains in the water can be measured. The oxygen used up or the BOD value can be calculated , for the particular water body.
Hardness
• Hardness isdue to dissolved metal ions, namely Ca2+ and Mg2+. Here the contribution of Ca and Mg are the most considered and other metals are negligible because they are less soluble in water. Therefore, we discuss Ca and Mg only here. Hard water has no adverse health effects. Hard water is less desirable because it requires more soap for cleaning. It forms scum and curd and it toughens vegetables during cooking and forms scales in boilers, hot water heaters and pipes. The composition of ground water naturally reflects the underlying geology, the residence time in the rock, the previous composition of the ground water and in some instances, the flow path. Due to the slower movement of ground water in the aquifers as compared to that of surface water, the composition of the former shows a negligible variation with time for a given aquifer
.
Ca2+(aq) / Mg2+(aq) + 2HCO3–(aq) → CaCO3(s) + MgCO3(s) + CO2(g) + H2O(l)
• Aquifer – A porous permeable rock layer containing water (Groundwater).
Iron (Fe)
• The primary source of iron in water is rock layers containing iron ore. Iron is typically dissolved in water and when brought to the surface, can form ‘rust’ which may settle out. Another source of iron is iron-reducing bacteria, which depends upon iron to live. The commonest iron containing water is red , laundry spotting, metallic in taste and staining of plumbing fixtures. These are usually due to the presence of iron above 0.3mg dm-3. Iron affects the taste of drinking water.
Fluoride (F–)
• Varying amounts of fluoride are found in groundwater of different areas of Sri Lanka. For example, due to the apatite ore in Eppawala fluoride, concentration of ground water in the surrounding areas is high. Fluoride can affect teeth during the period when permanent teeth are being formed. For tropical countries the fluoride content should not exeed 0.6 mg dm-3
Nitrates
• Nitrate is a common contaminant found mainly in ground water. High nitrate concentrations can be particularly dangerous to babies under six months, since nitrates interfere with the ability of blood to carry oxygen. Nitrate also causes cancer. Fertilizers, human and animal sewage are usually enriched with of nitrogenous compounds which may enter into a ground water body as a result of leaching. The conversion of ammonia to nitrate is brought about by highly specialized soil bacteria.
Phosphates
• Phosphate ions are added to water by chemical fertilizers and artificial detergents. Due to nitrates ions and phosphate ions, an eutrophication condition arises in water and enhances the growth of algae. As a result, amount of dissolved oxygen in water decreases.
Sedimentation
• The separation of a suspension of solid particles into a concentrated slurry. The supernatant liquid, after sedimentation is clear.
• If the effluent is suitable it will be discharged into waterways. Otherwise it is passed to a secondary treatment.
Coagulation
• Muddy river water in large water supply schemes can be coagulated using aluminium salt(alum).
• Water is stored in large tanks and can be coagulated with A1(III) or Fe(III).
• A gelatinous precipitate of aluminum hydroxide or iron(III) hydroxide is formed. As it settles and sinks to the bottom, the precipitate carries suspended material with it as sludge.
Flocculation and Filtration
• During the flocculation smaller particles are agglomerated to form bigger particles and these particles are filtered.
• After that water is passed slowly through sand filters. Different types of sand filters are used for water flow.
• Fine sand
• Course sand
• Gravel
• Stones
• Filtration removes microbes and other suspended particles from water. Many filters will also remove some harmful chemicals found in water.
Disinfection process
• Use of chlorine
Cl2, ClO2 ,chloroamines are used as disinfectants. They kill bacteria by oxidising. The residual Cl2 prevents the formation of further bacteria. But excess Cl2 reacts with organic substances and forms harmful substances including trihalomethanes and chlorinated phenols.
• Use of ozone
Ozone also destroys bacteria by oxidation. But it dissociates quickly. Ozone does not give further protection from bacteria. Therefore the water disinfected by ozone has to be used quickly. Since ozone doesn’t give any side effects it is preferably used by human. Unlike Cl2, ozone needs no storage and it can be generated easily.
• Use of UV radiation
It kills both the bacteria and viruses. As ozone it cannot have a further protection from bacteria.
Ti – Ilmenite FeTiO3 , Rutile TiO2
Fe – Hematite Fe2O3 , Magnetite Fe3O4 , Iron pyrites FeS2 , Siderite FeCO3
Cu – Chalcopyrite CuFeS2
• Iron ore, coke (as a reducing agent) and limestone (as a slag forming substance) are used as raw materials. The amount of CaCO3 used is dependent on the amount of silicate materials in the ore.

• Hot air is blown in at the bottom. Coke burns producing heat and CO.
C(s) + O2(g) → CO2(g) CO2(g) + C(s) → CO(g)
• The temperature at the point where air enters is at about 2000 K and at the top is about 700 K.
• The iron(III) oxide is reduced to iron mainly by CO and some by carbon.
• The molten iron containing 3-4% dissolved carbon forms pig iron. Melting point of the pure iron is 1535 °C but the melting point of pig iron is at about 1015 °C, due to the presence of impurities.
• CaCO3 decomposes to CaO and CO2 gas. CaO reacts with silicate impurities and forms slag (CaSiO3). Slag is also molten and floats on molten iron into the bottom. Slag protects iron from oxidizing by the air which is blown in the bottom.
• Pig iron contains 3-4 % of carbon and possibly other impurities such as Si, P, S and Mn.
• Following reactions are taken place in the temperature range of 700 K – 2000 K.
At low temperature (Below 1000 0C)
3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g)
Fe3O4(s) + CO(g) → 3FeO(s) + CO2(g)
FeO(s) + CO(g) → Fe(l) + CO2(g)
CaCO3 (s) → CaO(s) + CO2(g)
At high temperature (Above 1000 0C)
2FeO(s) + C(s) → 2Fe(l) + CO2(g)
CO2(g) + C(s) → 2CO(g)
CaO(s) + SiO2(s) → CaSiO3(slag)
CaO(s) + Al2O3(s) → Ca(AlO2)2(slag)